It’s not all about the Coronavirus
Though the COVID-19 pandemic has claimed the lives of over 120,000 people and is causing the suffering of almost 1.3 million patients in the United States alone, cancer and heart disease remain the leading causes of death in the country.
The American Cancer Society journal estimates that there will be around 1.8 million cancer cases this year, with 606,520 of those resulting in deaths.
Needless to say, the continuously increasing incidence of this deadly disease has prompted a number of companies in the biotechnology and healthcare sectors to invest substantially in creating and developing drugs for cancer treatment.
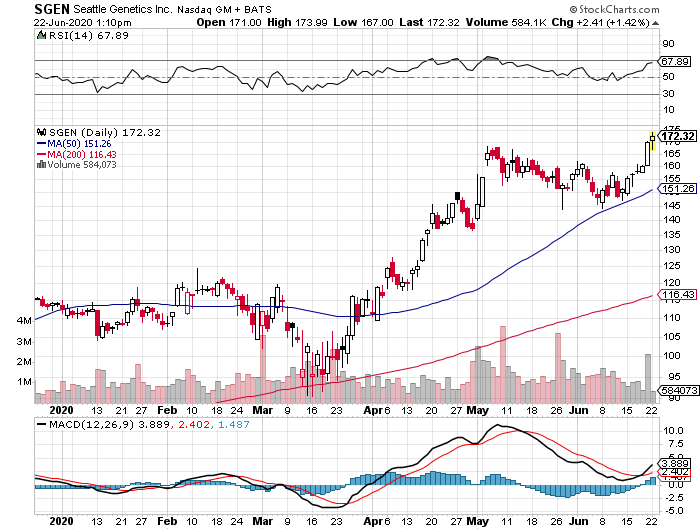
Buoyed by this demand, biotechnology company Seattle Genetics (SEGN) has gained 40.1% in 2020 so far primarily thanks to its cancer drugs.
In fact, Seattle Genetics welcomed 2020 with a newly approved drug called Padcev, which the company developed alongside Tokyo-based Astellas Pharma to treat the most common type of bladder cancer.
Despite the pandemic, Padcev sales have been exceeding expectations and analysts are jacking up the sales estimates for this potent bladder cancer product.
Initially pegged to rake in roughly $10 million in quarterly sales, Padcev managed to beat the estimates by four- to fivefold with $34.5 million in the first quarter of 2020.
Since then, peak sales prediction for this drug has been increased to a whopping $2 billion, with its 2020 sales target to be around $221 million.
Approximately 80,000 new bladder cancer cases are diagnosed every year in the United States. Among these patients, 90% suffer from the urothelial type -- the kind that Padcev is formulated to address.
Adding to that, Padcev’s success can also be attributed to the fact that it’s the only FDA-approved product for this particular patient set.
Riding on the momentum of Padcev’s unchallenged success in the bladder cancer field, Seattle Genetics and Astellas are now looking to expand the drug’s indication to cover an even larger patient set.
If this works out, then Padcev opens a whole new slew of possibilities to the tune of an additional $5.8 billion to its revenue.
At the moment, Padcev is also not prescribed to patients in the earlier stages of the disease - a demand that Seattle Genetics aims to address with its collaboration with Merck (MRK) via the immuno-oncology’s powerhouse drug Keytruda.
Aside from its bladder cancer drug, Seattle Genetics is also actively making a name for itself in another field.
In April 2020, Seattle Genetics received another positive news from the FDA.
The company’s breast cancer drug Tukysa, which was expected to gain approval by August this year, received the green light four months earlier instead.
Tukysa is another potential blockbuster drug for Seattle Genetics, with the product’s peak sales estimated to reach $1.2 billion by 2030.
All these are actually pretty impressive considering that Seattle Genetics was a one-product biotechnology company just a year ago.
Its single product, Hodgkin lymphoma drug Adcetris, had a specially impressive 2019 because of label expansions.
The drug posted a 32% jump in net sales to reach $627.7 million in the US and Canada. For 2020, Adcetris’ sales is expected to grow somewhere between 8% and 12%.
Apart from expanding the use of both Adcetris and Padciv, Seattle Genetics is also looking into developing new antibody treatments specifically for patients with solid tumors and lymphomas.
It currently has several candidates undergoing clinical trials, with some of these potential treatments expected to go head-to-head against active competitors in the space, including Roche (RHHBY), Novartis (NVS), Takeda Pharmaceutical (TAK), Pfizer (PFE), and Immunogen (IMG).
Prior to the approval of Padcev and Tukysa, the major growth driver that augmented Adcetris’ earnings was the company’s royalty revenue.
In the fourth quarter of 2019, the biotechnology company raked in $72.3 million in royalty revenue. This is actually triple the amount it earned in the same period in 2018.
The main source of its royalty revenue at the time is the $40 million in milestone payment it received from Takeda.
The payment was triggered by the annual net sales of Adcetris that went beyond $400 million in Takeda’s territory.
The total royalty revenue was also supplemented by a milestone payment from GlaxoSmithKline (GSK) and an upfront payment from Seattle Genetics’ work with Beijing-based company BeiGene (BGNE).
In the first quarter of 2020, royalty revenues jumped to $20 million compared to the $16 million the company earned during the same period in 2019.
Once again, this growth was attributed to Adcetris’ sales and boosted by royalties from the company’s collaboration with Roche (RHHBY) on the latter’s lymphoma drug Polivy.
Seattle Genetics has consistently grown its revenue since 2011 when its first-ever drug Adcetris received approval. With the recent additions of potential blockbusters Padcev and Tukysa, the company’s financial picture looks brighter than ever.
One of the key factors in its success is that the company addresses significant patient sets, providing its investors with the confidence that it can attract physicians and patients on board.
The Hodgkin lymphoma drug market, which Adcetris has covered, is anticipated to grow by roughly $1.24 billion from 2019 through 2023.
The urothelial cancer drug market, where Padciv is currently king, is estimated to hit $3.6 billion by 2023, with a 23% compound annual growth rate.
Tukysa addresses another patient set with high demand as well, with reports showing that the spending on HER2-positive cancer is anticipated to jump by 54% to hit $9.89 billion by 2025.