Innovation and establishing groundbreaking frontiers are never straightforward. This high-risk, high-reward endeavor is littered with skeletons of failure along the way.
The situation is particularly prevalent in the biotechnology and healthcare sector. However, there are a handful of companies that manage to navigate the risks.
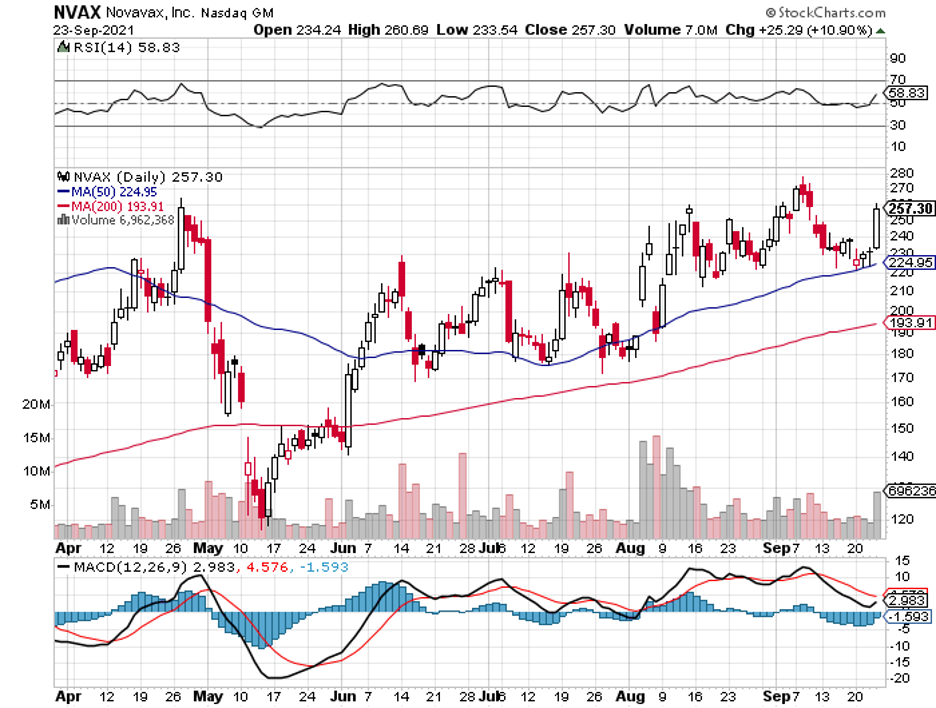
Novavax (NVAX) is one of them.
While the chance to become one of the early investors to buy Novavax at $5 has passed, I think the stock is still a good opportunity.
Right now, we’re at the foothills of its mid-phase potential—a few steps away from its high-growth stage.
At this point, what was previously assumed as a high-risk investment in Novavax can already be perceived as a calculated risk, and the reward can now be seen from a distance.
Simply put, there’s still a chance to invest in Novavax because its story is still being written.
So far, Moderna (MRNA) has a market capitalization of $183.50 billion, while BioNTech (BNTX) has $85.61 billion. In comparison, Novavax comes in positively cheap at $19.16 billion.
The key difference is that Moderna and BioNTech already have their COVID-19 vaccines out in the market.
As for Novavax, the biotech’s candidate, NVX-CoV2373, is still waiting for the green light for its first Emergency Use Authorization.
When government agencies begin letting Novavax distribute its COVID-19 vaccine, though, the stock is projected to soar.
The approval for NVX-CoV2373 is almost inevitable, as the vaccine showed an impressive 96.4% efficacy against the original strain—a higher percentage than Pfizer (PFE) and Moderna’s candidates.
If all goes well, the vaccine could be available by the fourth quarter.
As golfers would say, it’s only a chip and a putt from this point to get Novavax’s COVID-19 vaccine out in the market.
Given this projection, Novavax is expected to be the No. 3 COVID-19 vaccine distributor globally, easing out competitors Johnson & Johnson (JNJ) and AstraZeneca (AZN).
Despite not being a first mover in the COVID-19 vaccine race, Novavax can still seize a considerable market share.
The majority of the global population has yet to be vaccinated, and the company has already secured a deal for 2 billion doses in 2022.
On top of that, biotech has several agreements, including 100 million doses for the United States, 200 million doses for Europe, 150 million doses for Japan, and more than 1 billion doses for some developing countries.
In fact, the United States paid an advance of $1.3 billion for 100 million doses. That puts NVX-CoV2373 at roughly $13 per dose.
Although the purchase agreements are confidential, the prices for Japan and Europe are likely to be higher, while the developing countries will be given discounted rates.
At this rate, it’s possible that Novavax’s revenue in 2022 will be higher than its current market capitalization.
However, Novavax’s prospective path to becoming a high-reward investment will most probably come on the coattails of its COVID-19 vaccine, NVX-CoV2373.
Despite getting overtaken by Pfizer and Moderna in the COVID-19 vaccine race, there’s a critical area where Novavax has a massive headstart from its larger rivals: the COVID-19/influenza vaccine combo.
Not only does Novavax have several candidates well on their way to clinical trials, but the company’s combo vaccines also have the potential to outperform the majority—if not all—of its competitors aiming to market similar products.
So far, Novavax’s flu candidate NanoFlu appears to be a frontrunner as it meets all the primary endpoints set for Phase 3 clinical testing.
If everything falls into place, then Novavax would be able to bring a COVID-19/flu vaccine to market by 2025.
While three to four years may seem like a long time, keep in mind that neither Moderna nor Pfizer has a timeline for any potential product in this segment.
Although we don’t exactly know the future pricing for these combo vaccines, we can use the COVID-19 vaccine earnings of Pfizer and Moderna as guides.
Pfizer and BioNTech anticipate roughly $33 billion in revenue, while Moderna estimates more than $20 billion. Combined, that’s over $50 billion.
It’s evident that a combo vaccine would signify a multi-billion-dollar market, and obviously, more than one player would be taking a crack at it.
Nonetheless, the first to market could end up with the largest share—a fact that Pfizer turned into reality with its weeklong headstart over Moderna in the COVID-19 vaccine race.
While Novavax failed to keep up in this race, the company appears to be ready to seize its second chance to lead the way in the COVID-19/influenza combo vaccine race instead. Needless to say, this potentially blockbuster product could become an absolute game-changer.