Thanks to COVID-19, a new generation of biotechnology players are gaining more traction in the market these days.
For decades, the biotech industry has been notoriously difficult to break into.
However, the pandemic has served to level the playing field—and the small-cap biotechs are definitely taking advantage of the opening.
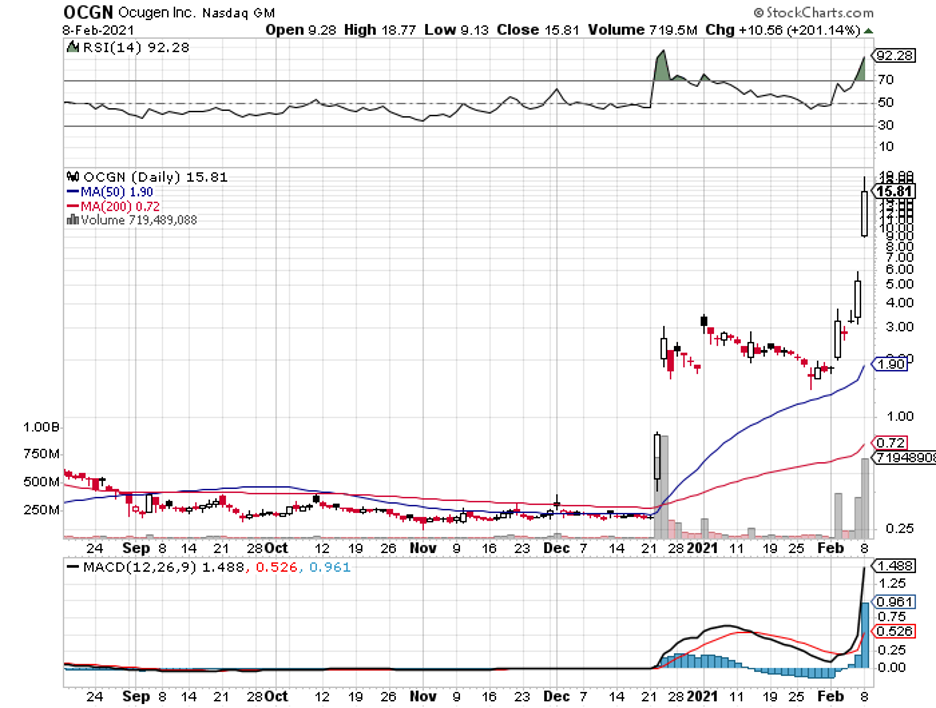
The latest to cash in on the opportunity is Ocugen (OCGN).
Founded in 2013, this Pennsylvania-based biotechnology company attracted media attention when it announced its partnership with India’s Bharat Biotech earlier this month.
The agreement between the two companies centered on Bharat’s COVID-19 vaccine candidate, Covaxin, which Ocugen plans to bring to the United States as soon as possible.
Ocugen will hold the US rights to Covaxin and be in charge of the development, regulation, and even commercialization within that market.
Even without any upfront payment, Ocugen will get 45% of the profits from the US markets in return.
While the news only broke this year, the partnership between the two has been months in the making, with Ocugen reaching a tipping point in December 2020 when it recorded a jaw-dropping 800% rise.
When this deal was announced, Ocugen shares were projected to rise from $4.50 to $8, showing off an already impressive increase from its measly 29 cents valuation less than a year ago.
Ocugen blew past those projections though as it’s now trading at $15 per share.
The kicker? The stock still has room to grow.
Realistically speaking, Ocugen shares can reach $20 to $25 after FDA approval this year. If its other candidates in the pipeline work out, then we can even see it hit $50 at some point.
Considering that Ocugen was able to sell 3 million common shares at a price that’s 46% higher than where the stock was when it closed last Friday, it’s clear that there’s a lot of optimism on the company these days.
The next question is this: Will Covaxin even gain approval in the US?
It can.
If it’s any indication, Covaxin has already been granted emergency use authorization in India.
Moreover, Covaxin was developed by Bharat, which is a highly reputable biotechnology company in India with 25 years of experience in the vaccine-making industry.
In fact, Bharat has developed over 16 vaccines and four bio-therapeutics, so it’s safe to say that the company knows its way around the business.
More than that, Bharat is confident that Covaxin can be effective against the new UK and South African variants of the coronavirus, making this vaccine candidate more potent than the other products under development today.
Unlike the vaccines of Pfizer (PFE) and Moderna (MRNA), Covaxin does not need ultra-cold freezers for storage. It can simply be stored at room temperature, making it a convenient option for a lot of distributors.
Prior to its deal with Bharat, Ocugen has been focused on its gene therapy which carries the potential to treat multiple retinal diseases with just one drug.
They call their breakthrough technology “one to many,” meaning the product could be the answer to several eye-related diseases.
Some of the rare conditions Ocugen has been working on are wet age-related macular degeneration, diabetic macular edema, and diabetic retinopathy.
Ocugen’s growth prospects, especially in 2021, heavily relies on the company’s ability to get an emergency use authorization for Covaxin in the US.
This means that investors should brace themselves for volatility in the next months as the vaccine candidate moves forward with the clinical trials.
In terms of long-term prospects, it remains to be seen how Ocugen will take advantage of the momentum it gained from the COVID-19 partnership with Bharat.