Outsmarting Opioids
Amid the stark realities of America's opioid crisis, with a staggering 80,000 annual fatalities due to overdose, the pharmaceutical industry is on the brink of a significant shift.
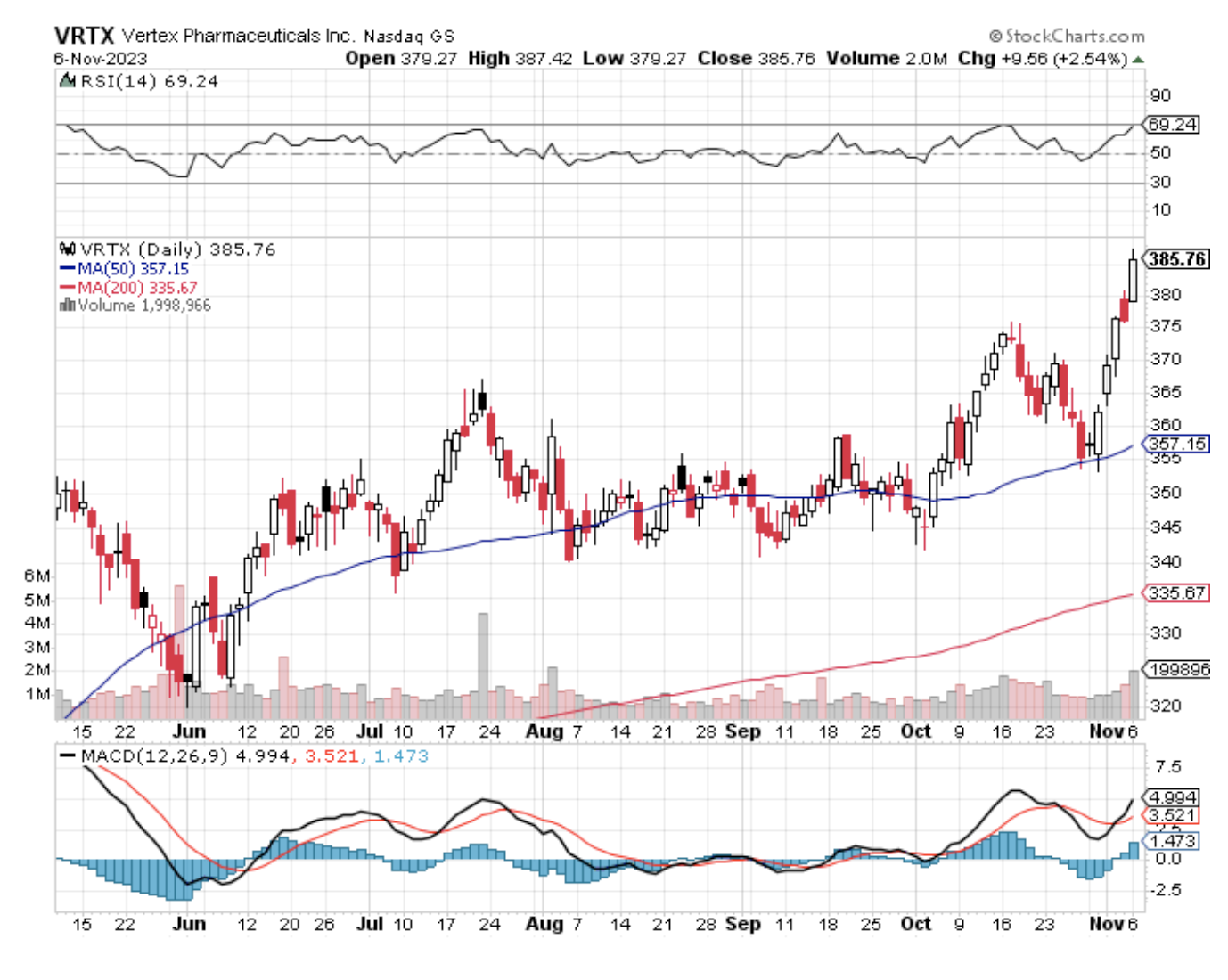
Vertex Pharmaceuticals (VRTX) stands out with its investigational drug VX-548, which promises a novel approach to pain management without the addiction risks of opioids. As the year winds down, this biotechnology company is poised to reveal findings from four clinical trials that could catapult VX-548 into the market spotlight.
Needless to say, the stakes couldn't be higher for Vertex.
The commercial success of VX-548, particularly in the chronic pain market, could mark a significant turning point. While generic opioids are cost-effective for short-term use, their potential for addiction and other risks make a non-addictive alternative like VX-548 an attractive proposition for insurers and patients alike.
Drawing parallels to the recent rise of GLP-1 obesity drugs by Eli Lilly (LLY) and Novo Nordisk (NVO), VX-548 could potentially mirror their impact.
Successful trials could see VX-548 generating annual revenues of $5.1 billion by 2030 — a substantial addition to Vertex’s current cystic fibrosis portfolio, which pulls in just shy of $10 billion.
Yet, it's essential to temper enthusiasm with a dose of reality. After all, the biotech sector is no stranger to the pitfalls of high expectations.
Past failures in the nonopioid pain sector underscore the importance of cautious optimism. Nerve growth factor inhibitors, once hailed as a breakthrough, faltered due to safety concerns, highlighting the unpredictable nature of drug development.
VX-548 aims to circumvent these issues with its unique mechanism of action that targets pain signaling at the peripheral nervous system—potentially a significant advantage over central nervous system-targeting opioids.
So, investors must weigh the risk-reward ratio of betting on Vertex ahead of these results.
This treatment’s success in acute pain management could result in a significant uptick in Vertex's stock value. Analyst projections suggest a potential increase of $58 per share if VX-548 matches opioid efficacy, with an $88 increase if it surpasses it. Should the chronic pain trials yield positive results, the stock could climb an additional $119 per share.
However, like I said, it's crucial to approach these numbers with caution. The market's response to trial outcomes can be unpredictable, and the memory of recent high-profile disappointments, such as Biogen's (BIIB) Aduhelm, still lingers.
In light of this, the downside should not be understated — a failed trial could see Vertex's stock take a substantial hit, potentially up to 20%.
Nevertheless, the financial health of Vertex remains strong even sans this pain management candidate. In fact, its top-selling TRIKAFTA/KAFTRIO patents are secured through 2037, accounting for a dominant 89.9% of sales.
This foundation provides a buffer against the inherent risks that come with drug development. With operating margins at a solid 45.6% and a GAAP EPS increase of 30.8% quarter over quarter, Vertex displays a financial resilience that may be reassuring to interested investors.
Taking everything into consideration, investors stand at a crossroads, with the potential of VX-548 offering both promise and uncertainty. The decision to invest now hinges on more than just the outcomes of the trials; it requires strategic consideration of the broader market, potential competitors, and the overarching trends in pain management.
As Wall Street watches with a trained eye, the early indications from Vertex’s trials suggest that VX-548 has a fighting chance to succeed where others have faltered.
If its subsequent tests affirm its potential, VX-548 could not only transform the company’s financial landscape but also mark a significant advancement in the fight against the opioid epidemic — a win for both public health and discerning investors. I suggest you buy the dip.