Revolutionary New Discovery Converts CO2 Into Alcohol
The Internet has recently been abuzz about a revolutionary new discovery that converts carbon dioxide (CO2) into ethanol.
Readers often refer me to new, incredible, and earth-shattering technologies which often turn out to be nothing more than investment scams. The cold fusion boom of the 1990s comes to mind.
As a reformed and non-practicing biochemist, I decided to check this one out.
What I discovered blew my mind.
Scientists at the US Department of Energy?s Oak Ridge National Laboratory literally stumbled across a process that could become a game changer for the entire energy industry.
Carbon dioxide is passed over a carbon and copper catalyst, energized with a jolt of electricity, and ethanol comes out the other end. How nice is that!
Ethanol (CH3CH2OH) is the kind of alcohol we drink, and is found in beer, wine, and spirits. It is not to be confused with methanol (CH3OH), known as rubbing alcohol which is lethal if drunk in quantity.
Ethanol boils at 78.4 degrees centigrade, compared to 64.7 degrees for methanol, which is how home distillers and moonshiners keep from killing themselves.
Oak Ridge National Laboratory is no slouch when it comes to energy research.
Built in Tennessee in a huge rush during 1942, it used to be one of the most secretive research institutions in the world.
Its original mission was to produce highly enriched uranium for the first atomic bomb, known as the Manhattan Project. Many of my college professors worked there during WWII.
In recent years, it has broadened its mandate to include materials science, artificial intelligence, systems biology, and national security. It possesses the Titan, one of the world?s most powerful super computers.
Since President Obama came into office, it has received an infusion of cash to explore new forms of alternative energy.
To visit their website, click https://www.ornl.gov .
The key to their method is how the copper is arranged. First, the researchers create a scaffold made from carbon and nitrogen. The surface is covered in tiny spikes, each about 50 nanometers high.
The researchers then deposit copper particles onto the surface which acts as a catalyst for the reaction.
When electricity is run through the material, the reactions are concentrated?at the very tips of the spikes, providing the energy required?for carbon dioxide dissolved in water to break apart and reform as ethanol.
The?reaction achieved an efficiency of 63 percent, using a power supply of just 1.2 volts at room temperature.
Of course, the process is still years away from scalability and mass production.
But the fact that this process can take place at room temperature and requires only small power inputs means it could become economically viable.
The long-term possibilities of this new technology would be momentous.
Previous carbon capture and conversion plans were wildly expensive. Carbon dioxide is one of the most stable molecules in the universe. Until now, breaking it apart required huge amounts of energy.
That is why the end products of all combustion are CO2 and H2O. There is nowhere else for a chemical reaction to go without help.
It would cost more than $1 trillion just to convert America?s existing coal fired power plants (to read more, click The Price Tag for Clean Coal).
This new technology could capture the carbon dioxide emitted by power plants, convert it into ethanol, and then burn the ethanol, all in a closed system. Nothing would reach the atmosphere.
Solar cells could be used to produce the ethanol during the day, which is then burned at night.
Ethanol could also be used to power cars. In most states about 10% of the gasoline you purchase at the pump is comprised of ethanol.
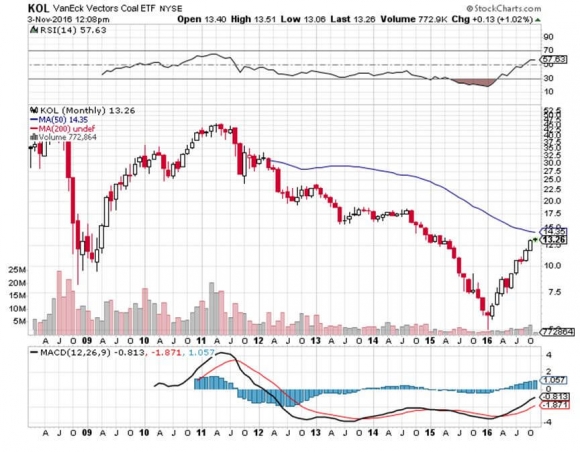
The new process could extend a lifeline to the beleaguered coal industry (KOL) which has been in free fall for years.
Many of the biggest firms, like Peabody Energy, Arch Coal, Alpha Natural Resources, and Walter Energy, have gone bankrupt.? It is unlikely that another coaled fired power plant will ever be built in the US again.
About 70% of America?s coal output is now exported to China, much of it in rail cars rumbling past my home late at night.
Coal's problems have become so severe that the plight of unemployed coal miners has become an issue in the presidential election.
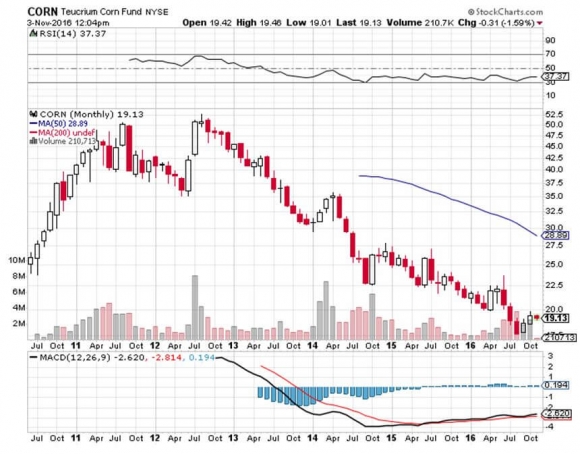
The big loser from this technological breakthrough (there is ALWAYS a big loser) is a farmer in the Midwest who grows corn (CORN).
About 40% of the total US corn production is now used to make ethanol for fuel, an activity heavily subsidized by the government (for more on that sensitive topic, please read The Great Ethanol Boondoggle).
If the carbon dioxide conversion process goes mainstream, which could happen sooner than you think, it could trigger a collapse in corn prices, by half or more.
Maybe that is what the price of corn has been trying to tell us. For a host of reasons, it has been one of the world?s worst performing asset classes for the past four years, down some 67%.
Mind you, converting carbon dioxide to ethanol is no panacea. Burning ethanol puts plenty of CO2 into the atmosphere as well, just not as much as gasoline. It is an improvement, but not a solution.
Sometimes, markets can sniff these things out sooner than we mere mortals can. And I can tell you that my nose, in particular, is finely attuned to the scent of alcohol.
Should Jack Daniels be worried?
To read more, click ?High-Selectivity Electrochemical Conversion of CO2 to Ethanol?