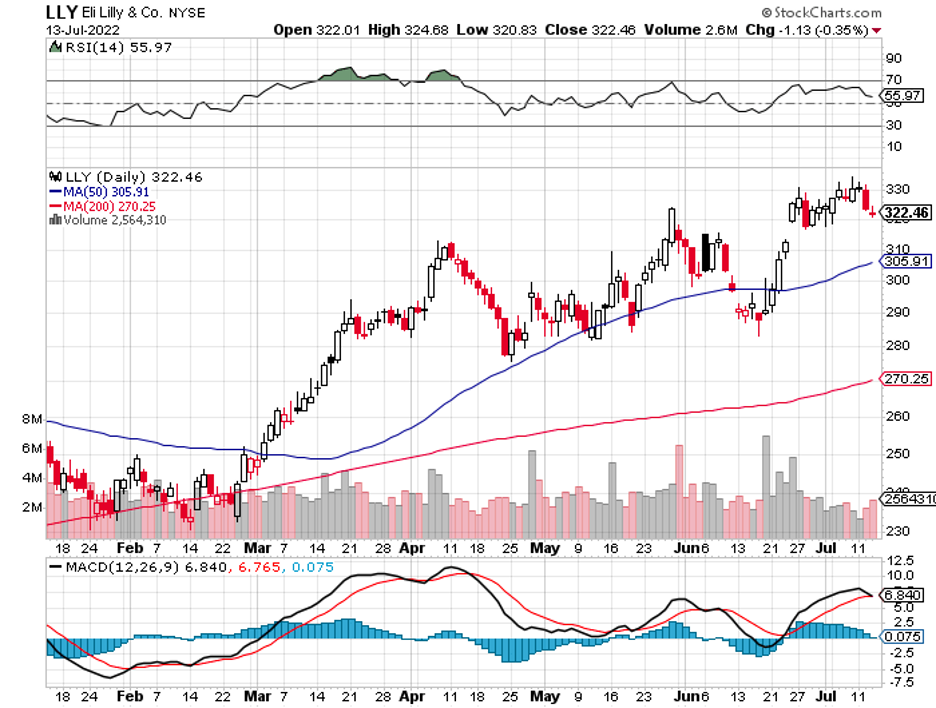
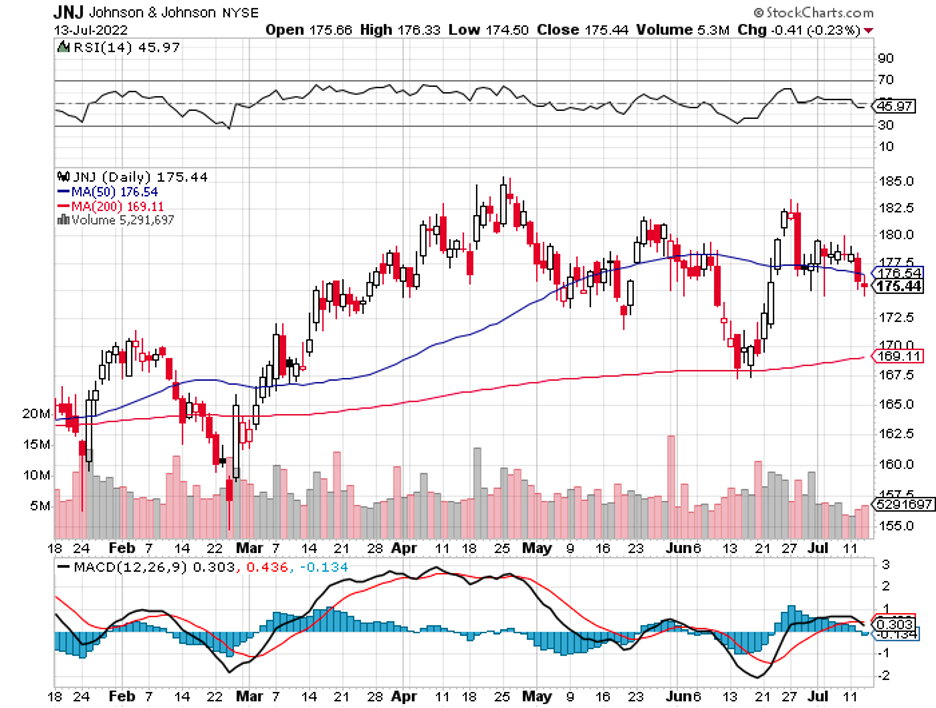
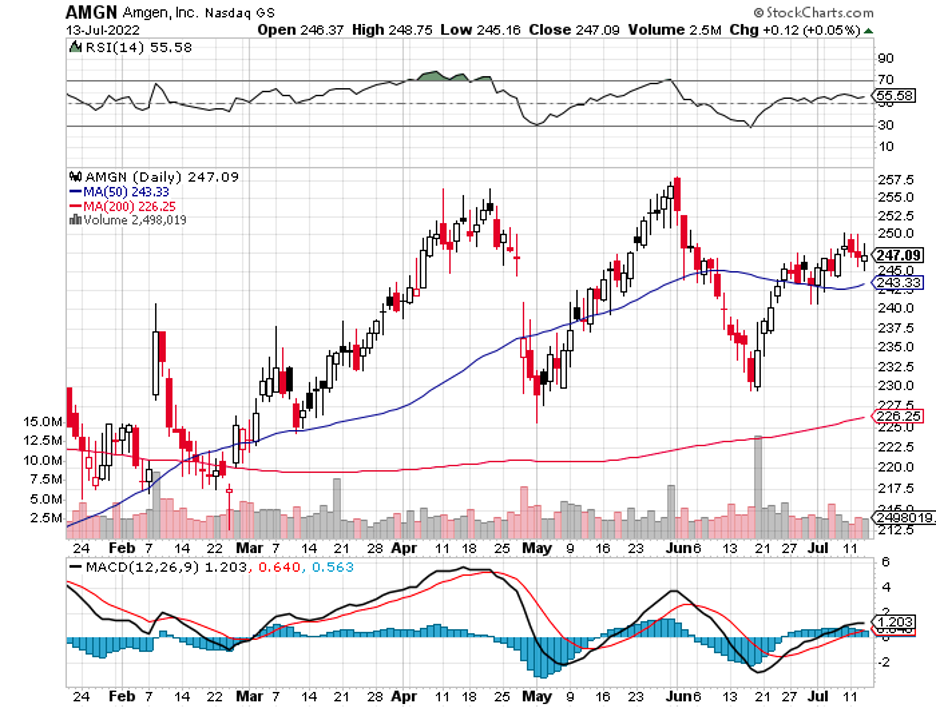
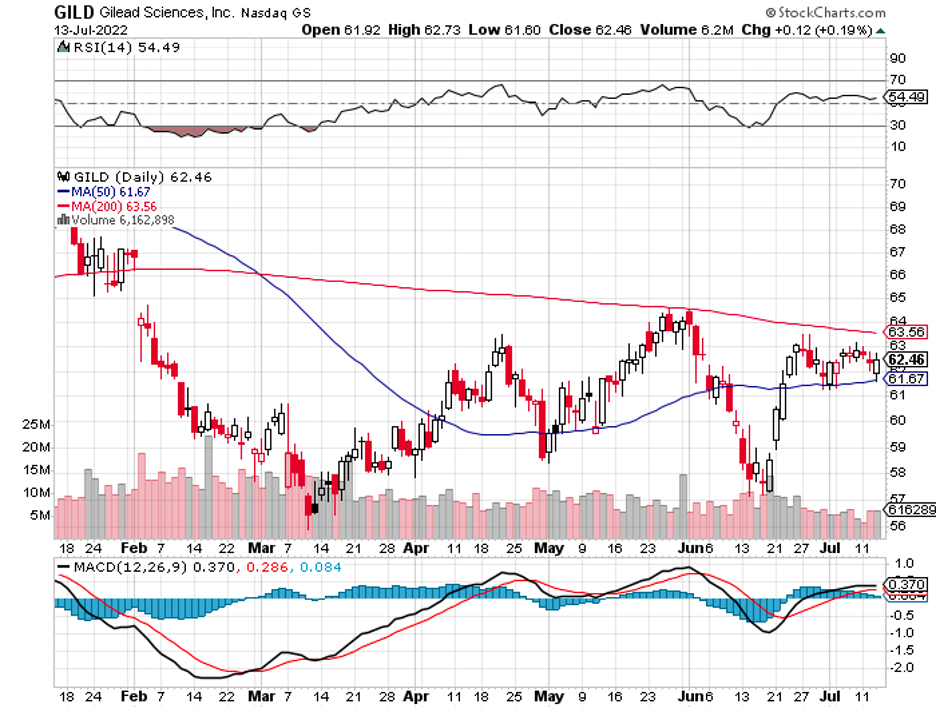
The broader market hasn’t been putting that much faith in drugmakers these days, and this could very well be a mistake.
While 2022 has not been particularly kind to equities recently, several names in the biotechnology and healthcare sector still managed to keep themselves safe from the selloff.
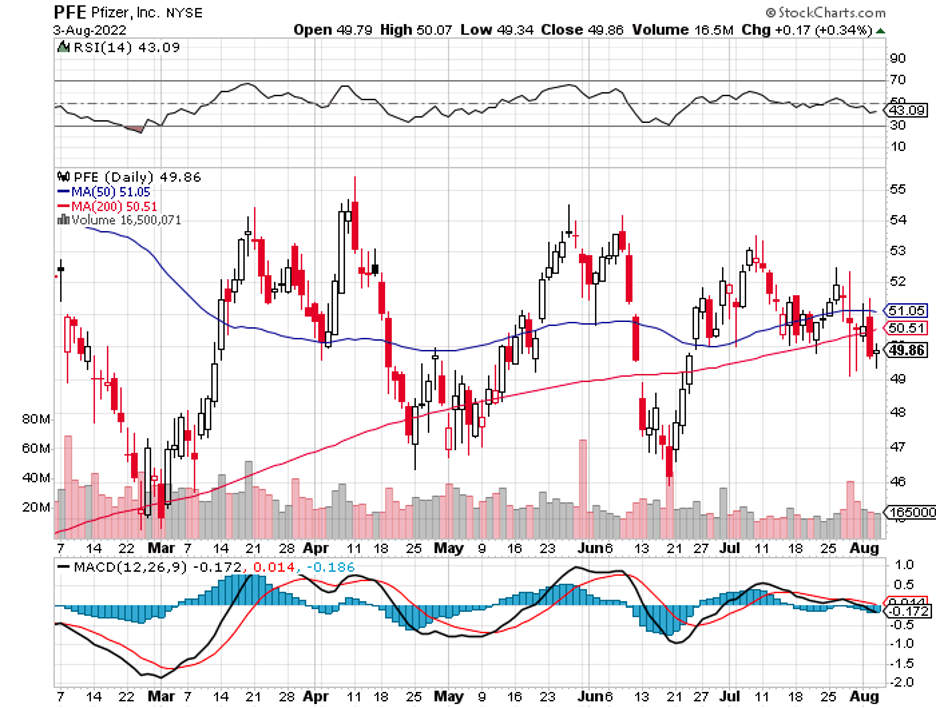
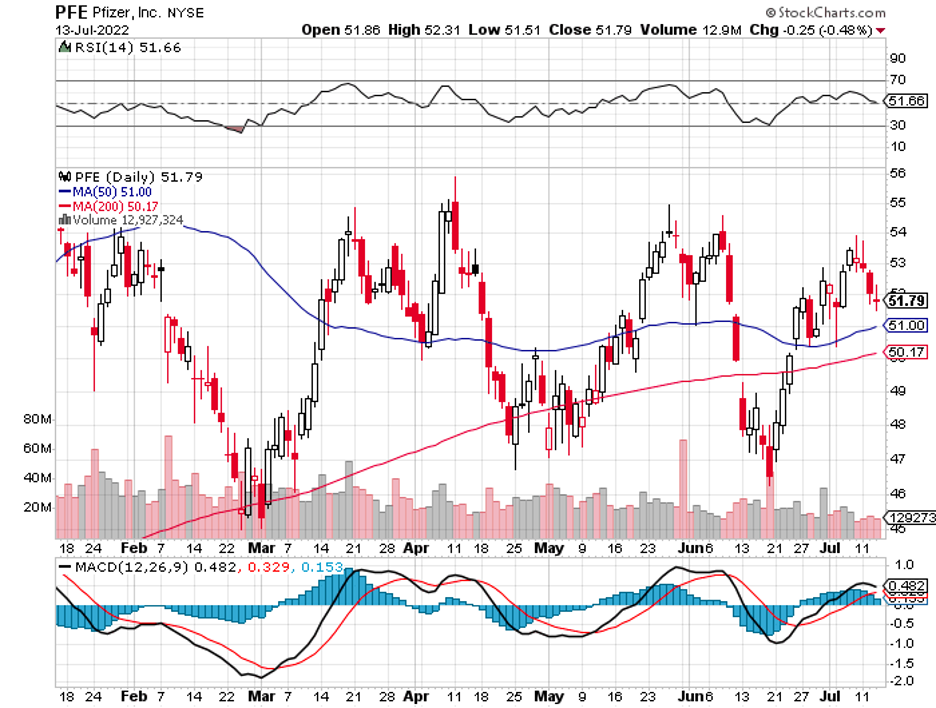
Pfizer (PFE), with its COVID vaccine sales, is one of them. Admittedly, this pharmaceutical giant has not shown substantial growth in the past monthS. Nonetheless, its quarterly updates and, more importantly, pipeline have exhibited notably encouraging signals.
As a massive underperformed in the past 20 years, Pfizer has taken aggressive steps to transform its strategy. The most obvious way to shake up the business is to eliminate the bulk of its noncore products.
However, it’s not advisable to buy a company just because it has been underperforming and would then be sold at lower prices. Instead, it is critical to determine whether there’s a catalyst.
For Pfizer, the catalyst was clear: COVID.
The company was and still is at the heart of the coronavirus vaccine drives and treatments—a position that’s projected to be sustained for years to come.
The company has made a fortune from this program, and it’s still reaping the rewards in a massive way.
In the second quarter of 2022, Pfizer’s revenue climbed by 53% year-over-year to reach $27.7 billion. Based on the company’s record, this is the most significant quarterly sales during this period to date.
For context, its COVID vaccine, Comirnaty, raised $8.8 billion in sales. This is 20% higher than its reported sales in 2021 over the same period.
Meanwhile, Pfizer’s new COVID therapy, Paxlovid, recorded $8.1 billion in sales. Taken together, Paxlovid and Comirnaty comprise over half of the company’s total revenue for the second quarter.
Leveraging these growth opportunities, Pfizer has been steadily expanding its pipeline.
To date, the company has roughly 96 drugs in its pipeline. Of these, 6 drugs are in registration, while 29 candidates are queued for Phase 3 trials. There are 31 drugs in Phase 2 and 30 more in Phase 1.
Pfizer’s candidates range from treatments for inflammation, immunology, oncology, vaccines, and internal medicine to rare disease therapies.
Among the treatments in its Phase 3 study, two have been identified to bring in billions of dollars for Pfizer potentially.
One is PF-06939926, which is a treatment for Duchenne syndrome. The other is PF-06928316, which is for Respiratory Syncytial Virus (RSV).
Globally, 1 in 3,500 to 5,000 males suffer from Duchenne syndrome. This puts the number of patients at roughly 250,000, with about 10,000 to 15,000 found in the US. While it generally affects males, it can sometimes affect females as well.
In terms of market size, the Duchenne syndrome market is expected to be worth $4 billion in 2023 and $7 billion by 2027.
Currently, the major approved treatments for this condition are Sarepta's (SRPT) Exondys 51, Vyondys, and Amondys, as well as PTC Therapeutics (PTCT) Emflaza and Translarna.
PTC recorded $236 million in sales for Translarna, which is approved in Europe, and $187 million for Emflaza, approved in the US, for a total of $423 million in sales in 2021. Meanwhile, Sarepta’s overall sales reached $612 million for that same period.
Adding the rest of the minor competitors for Duchenne syndrome treatments, only $1.5 billion of the projected market value is held by the existing drugs. Clearly, there’s a lot of room for more companies to join the fray.
Meanwhile, RSV presents another lucrative market. According to the Centers for Disease Control and Prevention, this condition causes approximately 58,000 hospitalizations annually in the US.
Of these, 100 to 500 deaths are children under 5 years old and 14,000 are adults aged 65 and above. The average expense in managing adult patients alone has reached roughly $3 billion every year.
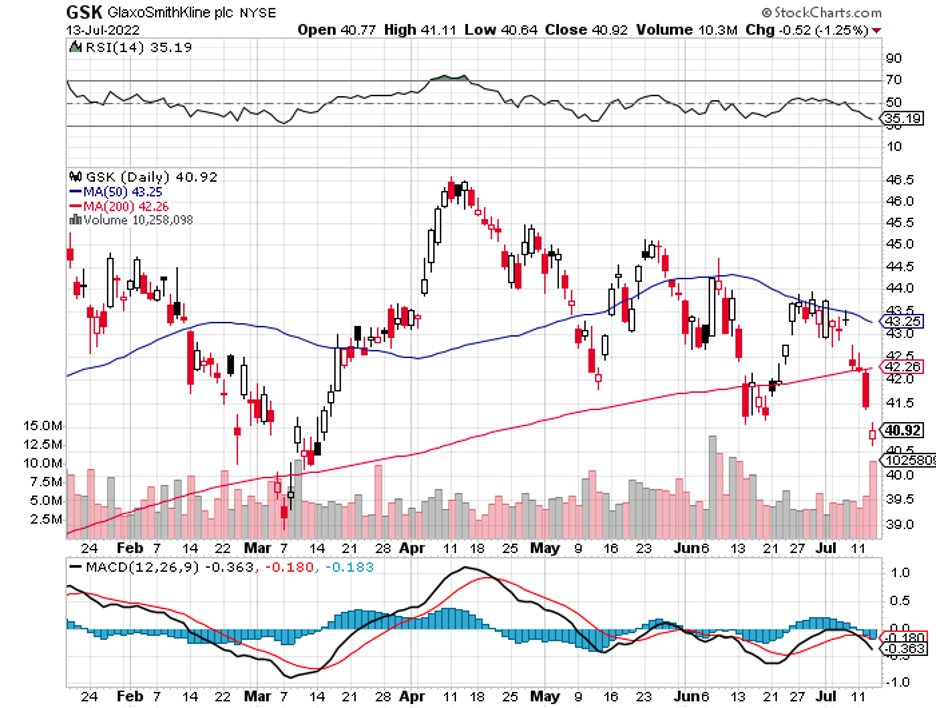
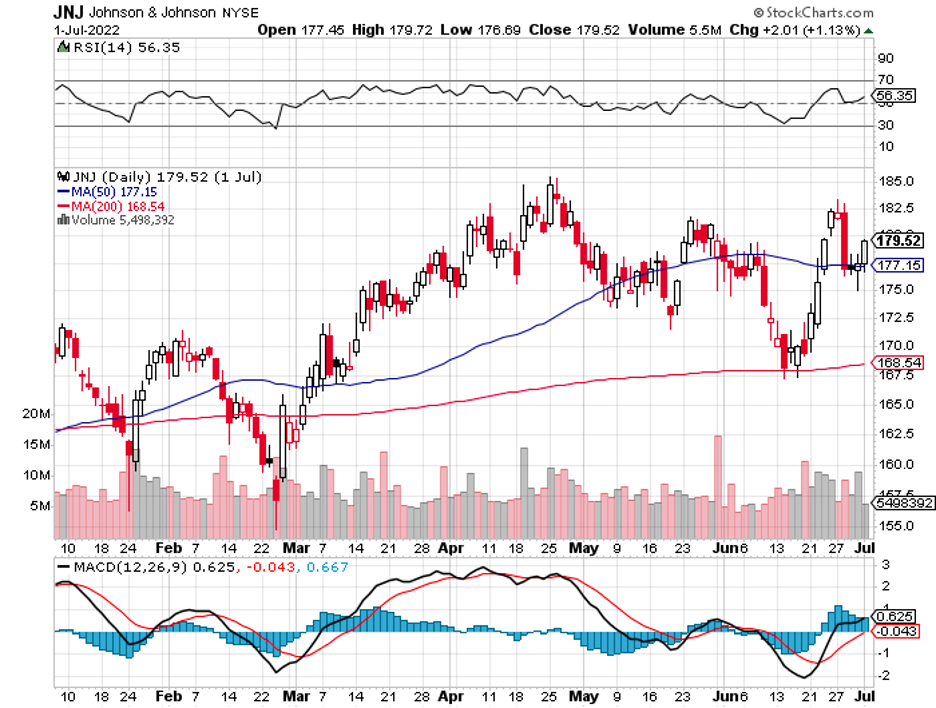
In terms of market value, the RSV market is projected to reach $4 billion by 2027. So far, the biggest competitors in this space are GlaxoSmithKline (GSK), Johnson & Johnson (JNJ), and Moderna (MRNA).
While its rivals are challenging, Pfizer still estimates sales for its RSV vaccine to reach at least $1.5 billion annually.
Thanks to its COVID programs, Pfizer has been hailed as the undisputed leader of the pack in terms of reputation and credibility in research.
Needless to say, these factors would serve as a valuable growth lever for the healthcare giant for decades.
As one of the largest biopharmas in the world, Pfizer has established a reputation for outstanding innovation. Over the years, the company has delivered several revolutionary treatments to the market like Viagra or Lyrica.
Simultaneously, it developed Lipitor, reaching $14.5 billion in sales over 14.5 years.
Since then, it has become a highly reputable industry name. Its diverse and extensive pipeline demonstrates that it remains a company highly capable of innovating and maintaining its dominance.