What truly scares investors isn’t really the potential relapse of the financial metrics that would undoubtedly take center stage again since the earnings season already commenced.
As we have been recently reminded, the world’s worst nightmares still place the COVID-19 virus front and center—this, despite the pandemic supposedly already under control.
COVID cases are starting to rise again, and the delta variant is seen as the latest addition to the problem.
That strain has actually become dominant in the United States, sending unvaccinated individuals to hospitals faster than its predecessor.
This delta variant is said to be more contagious than the previous virus. In fact, the fear over this new wave is incredibly high that the US government decided to issue a “Do Not Travel” advisory for the UK, which has been suffering from outbreaks despite their high vaccination rates.
If this is yet another indication of another disastrous year like 2020, then stocks would most certainly fall.
However, stocks managed to bounce back after the initial scare.
While this sparked a debate on the reason behind the recent sharp losses, investors are encouraged to remain focused on how the virus can still affect their lives despite the strong temptation to believe that the stock market will continue to rise from here.
Vigilance is the key to survival these days. It’s critical to bear in mind that the virus that upended our world and forced us to shutter our economies may not be in retreat just yet.
After all, even paranoids have enemies.
That’s what makes the mRNA vaccines a promising answer to this new issue.
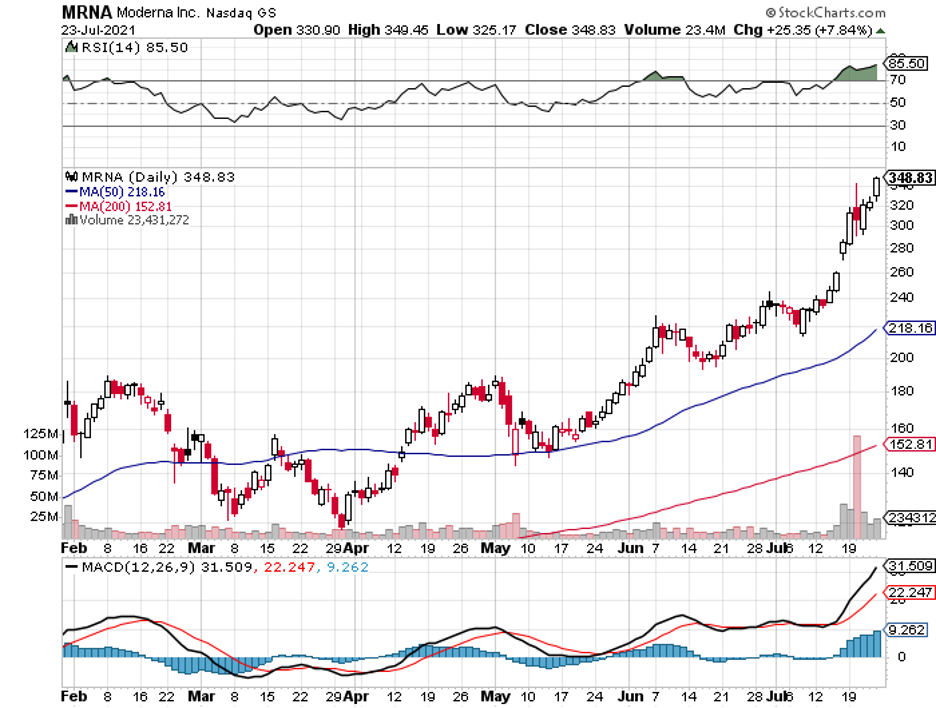
Moreover, against this volatile environment, one stock looks to be extremely intriguing: Moderna (MRNA).
Amid the analyses and reports, the most consistent thing about Moderna—and the most notable quality it has—is its solid science.
At its core, mRNA technology can transform the cells in a person’s body into mini-factories with the ability to produce virtually any kind of protein that Moderna wants.
So when people get inoculated with mRNA-1273, the mRNA instructs the body’s cells to start producing inactive replicas of COVID-19 proteins.
Then, the body’s immune system responds to those replicas.
This process effectively teaches the person’s immune system how to protect itself and fight off any type of exposure to the COVID-19 virus proteins.
Moderna’s vaccine is based on mRNA technology, which is primarily an information molecule. This means that this could be used to create various products, which would reach better technical success over time.
Basically, one major benefit of mRNA is that the chemistry behind the formulation for treatments like the flu shot uses exactly the same when creating more advanced products like the COVID-19 vaccine or even the HIV vaccine.
This makes it easier for Moderna to keep developing vaccines and other treatments because the company no longer needs to implement major changes in its manufacturing system for every new drug.
This is incredible leverage for Moderna, particularly across its manufacturing infrastructure and R&D. With the money saved on this advantage, Moderna can make massive investments in other segments like IT and marketing.
In fact, their quickness to market their vaccine is one of the reasons for Moderna’s success in the COVID-19 vaccine race.
Over the course of the last 52 weeks, Moderna stock has performed within the range of $54.21 to $342.51. The stock also managed to sustain its momentum from the surge of investor interest last year and is up by 207% so far this 2021.
In ensuring that it doesn’t get too far behind the leader, Pfizer (PFE)-BioNTech (BNTX), it secured a huge chunk of the market share as well.
Now, there’s a new virus strain threatening to take down everything we’ve worked hard to rebuild since the first COVID-19 case broke last year. This could mean an additional revenue stream for Moderna considering that it can deliver at lightning speed compared to other developers.
Aside from its work on COVID-19, Moderna is also looking for ways to use mRNA technology to develop treatments for heart failure, cancer, and other severe conditions.
To date, Moderna has roughly 24 mRNA-based programs in its pipeline, ranging from the Zika vaccine to cancer treatments.
Admittedly, buying Moderna stock presents risks. However, the greed and fear that continue to rule the markets places Moderna in a unique position to be the answer to all the questions.
Moderna is estimated to rake in $18.8 billion in revenue for 2021, thanks largely to its COVID-19 vaccine, with the number expected to rise as the company ramps up production to reach 3 billion doses in 2022.
Moderna’s dominance today doesn’t necessarily mean it’ll last forever, especially with competition coming in from Johnson & Johnson (JNJ) and Novavax (NVAX).
However, the technology it uses and the fact that it’s one of the only two biotechnology companies that successfully executed the mRNA place Moderna in an extremely unique, profitable, and secure position for the foreseeable future.