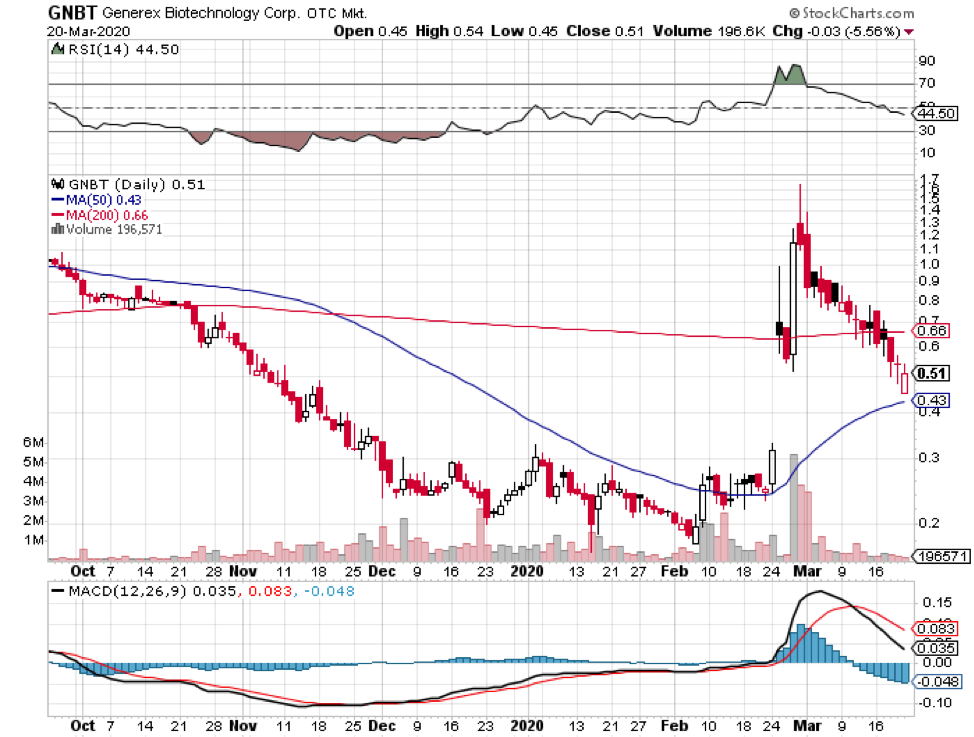
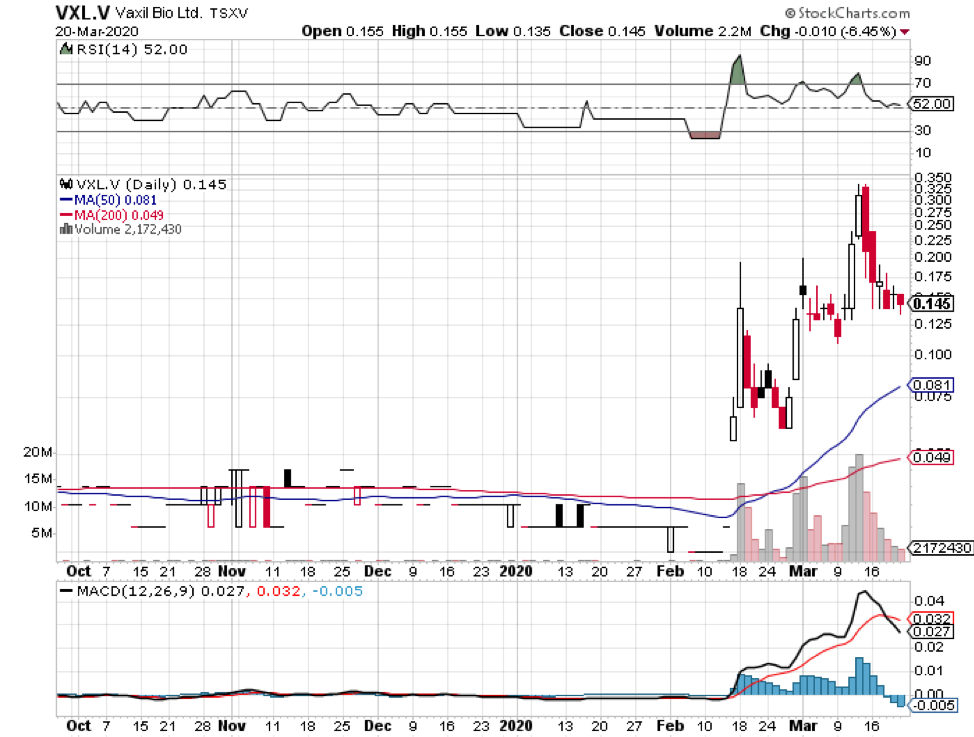
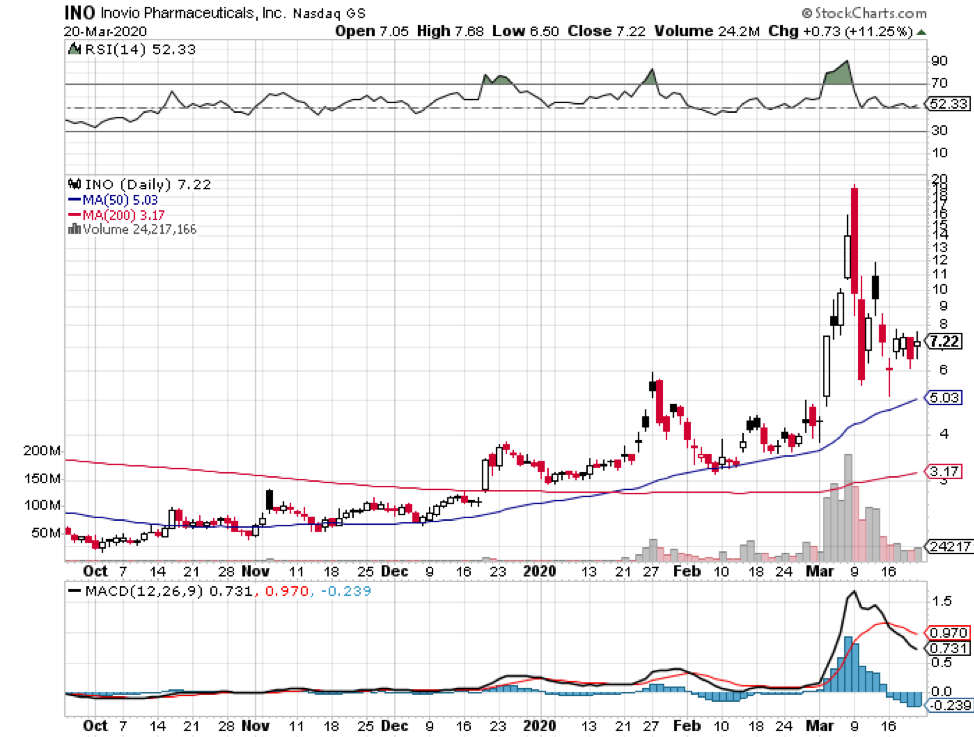
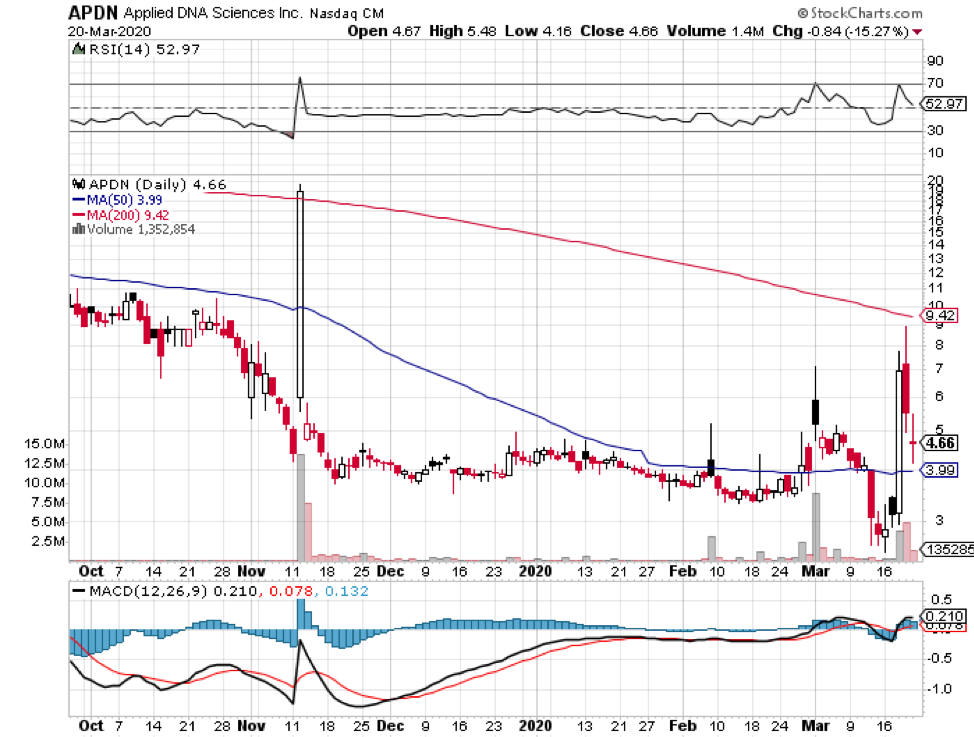
Among hundreds of companies working on a coronavirus disease (COVID-19) vaccine, the US Government has picked five companies as the most likely candidates to develop the much-needed immunization shot soon.
This is a part of a process that usually takes years and even decades to complete. The goal is to have a COVID-19 vaccine available for Americans by January 2021.
The decision to winnow the field even before final results are out is the administration’s way of focusing its energy and resources on the most promising vaccine candidates, thereby coming up with a solution faster.
Four of the five companies are based in the United States and one is from the United Kingdom.
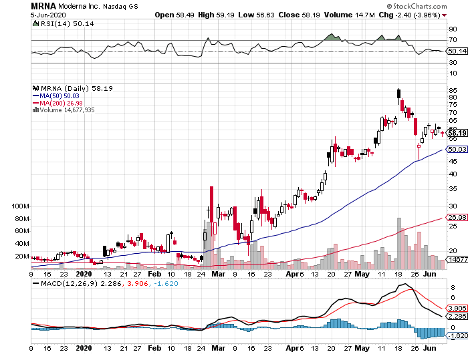
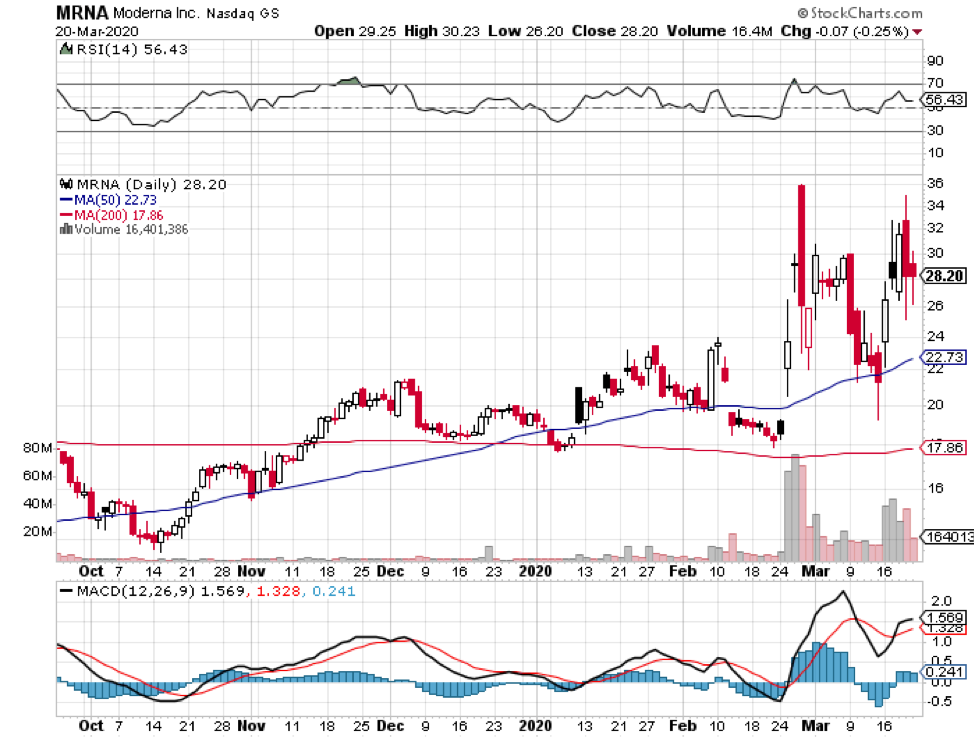
The list includes Massachusetts-based biotechnology firm Moderna (MRNA), which has a market capitalization of $22.63 billion.
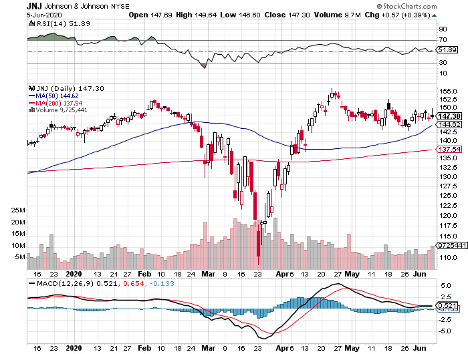
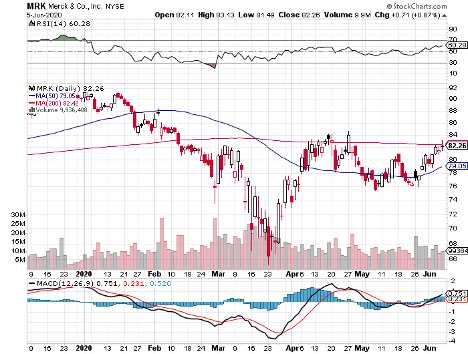
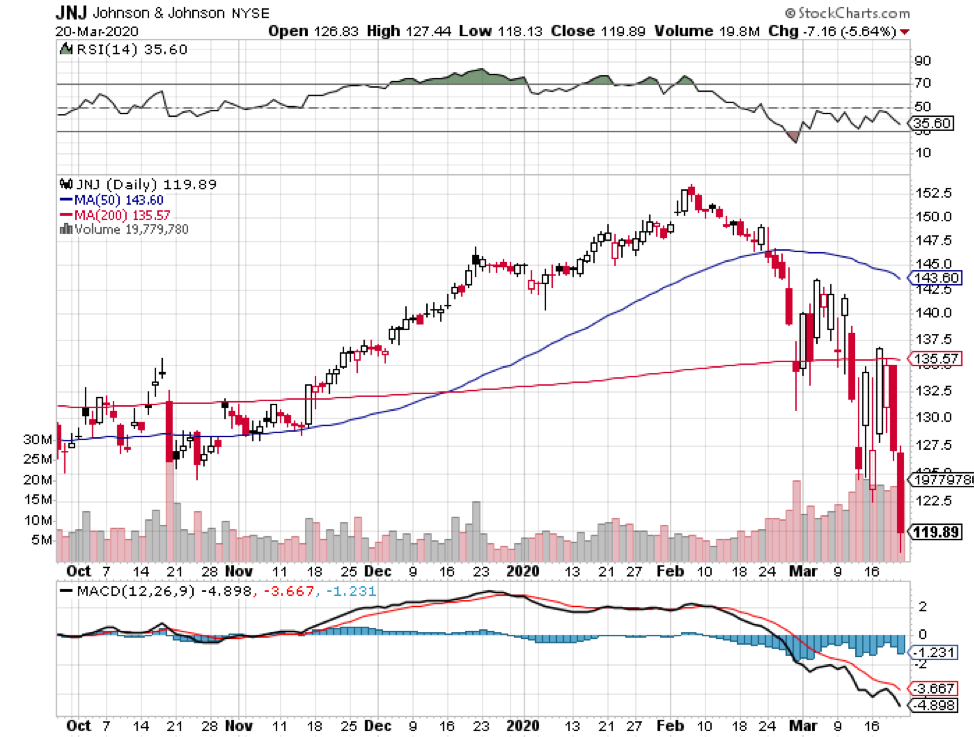
It also features biotechnology and healthcare giants Johnson & Johnson (JNJ), with its $388.08 billion market cap; Merck & Co. (MRK), which has $207.63 billion in market cap, and Pfizer (PFE), with a market cap of $199.92 billion.
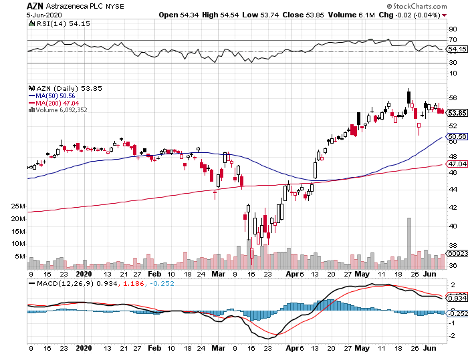
Cambridge-based pharmaceutical and biopharmaceutical company AstraZeneca rounds up the list.
Both Moderna and AstraZeneca are already in Phase 2 trials, which means the companies are testing their candidates on human subjects.
Looking at their timeline, the two would most likely move forward to Phase 3, which involves large-scale human trials, in July.
The Phase 3 trials will require roughly 30,000 participants for each vaccine candidate. If all five vaccine candidates reach Phase 3, then that means 150,000 people will be asked to participate as test subjects.
What we do know so far is that the agreements involve commitments from the biotechnology companies regarding intellectual property, the number of doses expected, and the estimated price limits.
Here’s a brief background of the top five companies under Trump’s COVID-19 vaccine radar today.
Moderna (MRNA)
Moderna’s vaccine, called mRNA1273, is undergoing Phase 2 trials. When news broke about Moderna’s progress with the COVID-19 vaccine, shares of the company exploded by more than 200% year-to-date.
For its Phase 2 trial, Moderna seeks to enroll 600 healthy individuals to test mRNA-1273 administered 28 days apart.
Throughout the COVID-19 crisis, Moderna has been a clear favorite of NIH’s Dr. Anthony Fauci.
He called the vaccine “quite promising” and described the results of the Phase 1 study to be “better than we thought.” What we know about the vaccine is that it can “neutralize” the virus in patients.
In terms of its release, Moderna is projected to deploy mRNA-1273 by the end of 2020.
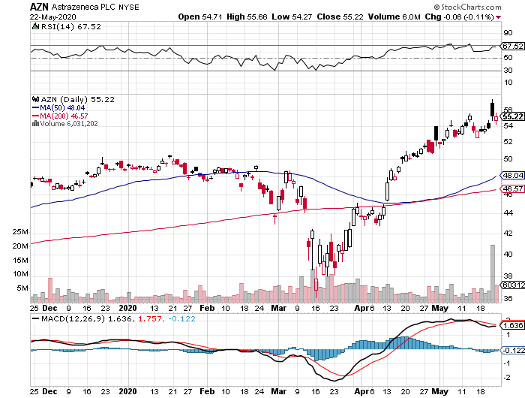
AstraZeneca (AZN)
AstraZeneca joined forces with Oxford University to develop AZD1222, which is now undergoing clinical trials in many sites in the UK.
Although the two have yet to complete its trials, AstraZeneca already agreed to supply 400 million vaccine doses to both the US and the UK in May.
Earlier this month, the company again completed a $750 million agreement with the Coalition for Epidemic Preparedness Innovations (CEPI), Gavi the Vaccine Alliance, and the Serum Institute of India (SII) to provide 1 billion vaccine doses to low and middle-income patients.
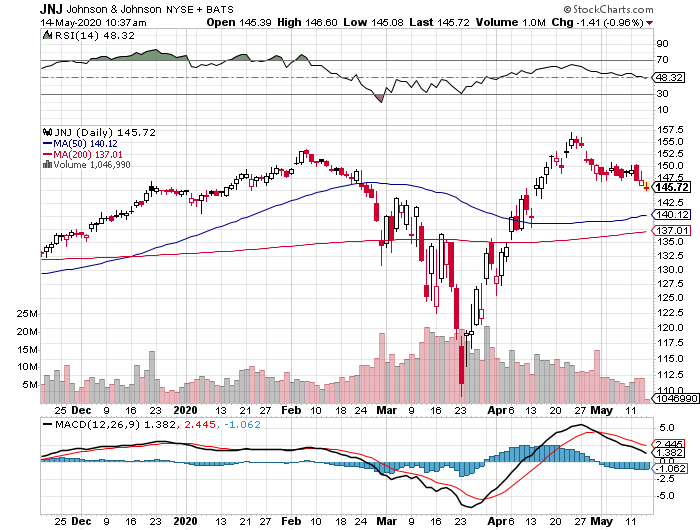
Johnson & Johnson (JNJ)
Johnson & Johnson aims to begin its Phase 1 clinical trial by September, with the ultimate goal to supply over 1 billion doses of COVID-19 vaccine across the globe.
Although Moderna and AstraZeneca are ahead in terms of vaccine development, JNJ has been impressing investors with its efforts outside COVID-19.
In the first quarter of 2020, the healthcare giant showed off a 3.3% year-over-year jump in its sales and a 54.6% increase in its net earnings.
The revenue of its pharmaceutical division rose by 8.7% while its health division saw a 9.2% increase.
Dubbed as the “Dividend King” in the industry, JNJ stayed true to its title as it continues its 58-year streak of raising its quarterly dividend.
Reports show that the company raised its quarterly dividend by 6.3% to reach $1.01 per share, reaping a solid yield of 2.73%.
Regardless of its performance in the vaccine race, JNJ has proven its resilience not only in the COVID-19 crisis but also in past crises like the dot-com bubble and the collapse of the housing market.
Merck (MRK)
Merck’s strategy is to build on the technology of its successful Ebola vaccine and establish partnerships with non-profit research groups.
Like JNJ, Merck is also a stable dividend stock that investors can buy and hold for years. In the past 10 years, this biotechnology leader has posted a profit, even managing to hit double-digits the majority of the time.
This is a trend Merck once again showcased in the first quarter of 2020.
In its latest report, the company showed off $3.2 billion in profit in sales worth $12.1 billion — demonstrating a decent profit margin of 27%.
Sales increased by 11% year over year, with cancer drug Keytruda heading the charge with its 45% revenue growth from the same period in 2019.
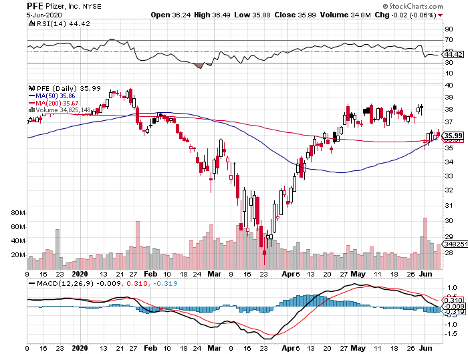
Pfizer (PFE)
Pfizer has been collaborating with German drugmaker BioNTech (BNTX) to develop BNT162.
The pharma giant is expected to have the vaccine candidate ready by October this year and be able to produce “hundreds of millions” of COVID-19 doses by 2021.
Although Pfizer and BioNTech joined the race later than Moderna, the big healthcare company’s edge is that it’s actually working on four vaccines simultaneously.
Simply put, this strategy offers them more than a single change of winning.
Along with the other three big biotechnology companies, Pfizer is a safe bet for those looking to invest in cutting-edge vaccine efforts but don’t feel comfortable risking it with a clinical-stage firm.
Like JNJ and Merck, Pfizer’s vaccine work sounds promising, but even if its COVID-19 program falters, the healthcare giant can still make a strong case as an excellent investment.
In its first-quarter report for 2020, Pfizer’s biopharma arm indicated an 11% jump, thanks to top performers like blood clot treatment Eliquis whose sales climbed by 29% to reach $1.3 billion.
Breast cancer medication Ibrance also contributed $1.2 billion, showing off a 10% year-over-year growth while Xtandi sales increased by 25% year over year to record $209 million.
Aside from these, Pfizer is hard at work in spinning off its Upjohn unit to combine with Mylan (MYL). This deal will guarantee Pfizer shareholders with 57% share of the new company called Viatris.
Just a few weeks ago, Trump compared Operation Warp Speed to the Manhattan Project, which was a government-initiated program that led to the development of nuclear weapons in World War II.
However, critics say that the “Skunk Works” initiative in California is a more fitting comparison for this COVID-19 effort. That is, the government could simply be wasting its resources on candidates that might never be able to leave the design stage.
Regardless of where you stand on Trump’s Operation Warp Speed, the fact remains that countless biotechnology and healthcare companies — big and small — are working on a COVID-19 vaccine.
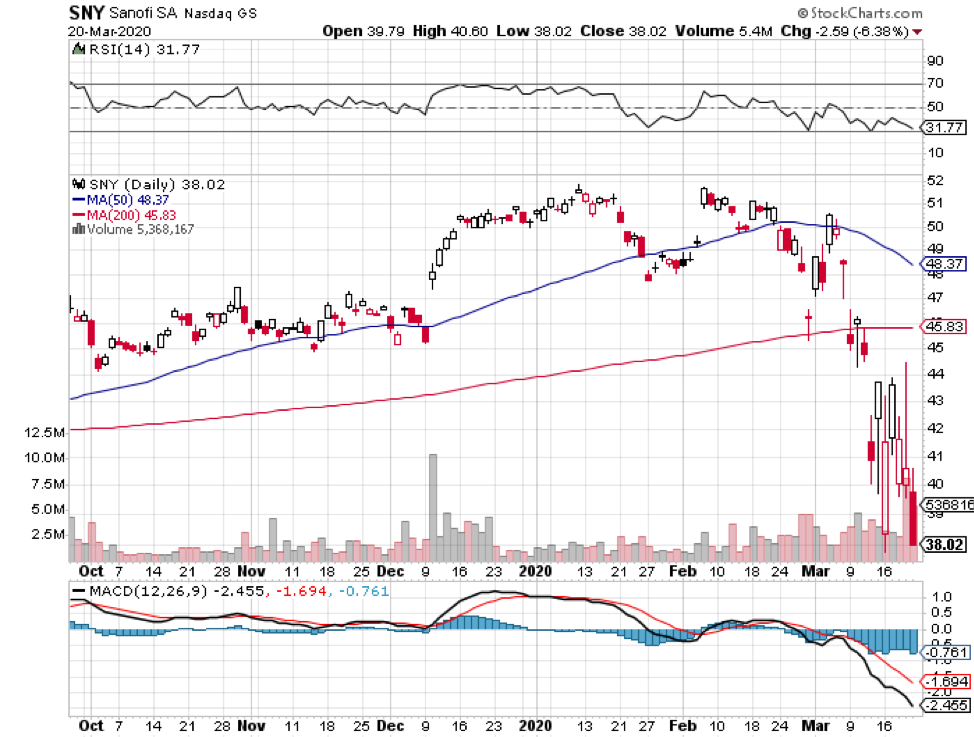
Outside the five companies chosen by the Trump administration, the list of strong contenders includes GlaxoSmithKline (GSK) and Sanofi (SNY).
Even smaller biotechnology companies like Inovio (INO) and Novavax (NVAX) are going all out on this.
Of course, it would also be foolish to completely disregard CanSino Biologics, which has been giving Moderna a run for its money since Day 1.
Despite not making the cut, these biotechnology and healthcare companies are still in hot pursuit and it’s still very much a neck-to-neck race.