The biotechnology and healthcare sector has been ruthlessly hammered in 2021.
In fact, the largest exchange-traded funds that keep track of the biotechnology industry have been in the negative in the past months.
However, the string of bad news doesn’t automatically mean that none of the biotechs can deliver strong returns in the coming days.
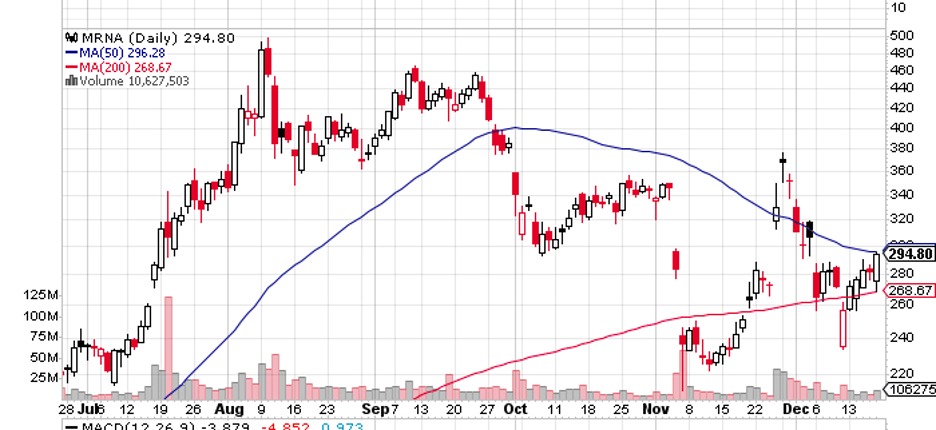
An excellent example of a biotech that’s an exception to the general theme of the sector these days is none other than the famous Moderna (MRNA).
Moderna stock has already delivered a 434% gain in 2020. Meanwhile, it has so far recorded a 160% rise this year—a number that’s expected to go higher before 2021 ends.
These gains came after the biotech became one of the market leaders in the COVID-19 vaccine race, alongside Pfizer (PFE) and BioNTech (BNTX).
Considering how COVID-19 catapulted the stock to dizzying heights, some investors fear that Moderna’s performance will decline in a post-pandemic setting.
That’s not necessarily the case.
Viruses present complex problems. Right now, we’re dealing with yet another coronavirus variant, Omicron.
This latest strain appears to be more contagious than the previously discovered Delta variant, which was then reported to be more virulent than the original.
What’s the takeaway here?
COVID-19 isn’t going to disappear anytime soon. Since the vaccines and boosters seem to wane gradually, these are expected to become staples moving forward.
This means everyone will need ongoing protection, which translates to ongoing sales for vaccines and boosters for companies like Moderna.
Moreover, the continuous demand for new and more potent vaccines makes it a no-brainer that Moderna will once again deliver market-crushing performances in the next few years.
For context, the company estimates that Spikevax, its COVID-19 vaccine, will rake in roughly $15 billion to $18 billion in sales in 2021.
Orders for 2022 have been secured as well, with Moderna already locked in for over $22 billion worth of Spikevax doses through advance purchase deals.
This is still expected to rise, considering the vaccines under development for the new variants getting discovered.
But even when the panic and anxiety over the viruses subside, we can still reasonably expect roughly $15 billion in annual sales from Spikevax
After all, the vaccine and boosters are expected to become the norm eventually.
Believe it or not, though, the best reason to buy Moderna isn’t its coronavirus vaccine.
Outside Spikevax, Moderna has a long list of promising pipeline candidates under development—the majority of which are based on the mRNA technology that’s behind its potent COVID vaccine.
While that does not guarantee that all the candidates will gain approval, the fact that the technology has been proven to work on humans presents a bright future for these candidates.
The company has been actively advancing its programs using its cash on hand, with over half a dozen queued in Phase 2 trials.
A potential blockbuster is its cytomegalovirus (CMV) vaccine candidate.
CMV, a virus that can be deadly to unborn babies and individuals with compromised immune systems, currently has no vaccine.
This represents an untapped market with high demand. Conservatively speaking, Moderna can generate roughly $2 billion to $5 billion in peak sales for this vaccine if it gains regulatory approval.
Other impressive programs in the biotech’s pipeline are its HIV vaccine candidate and a personalized cancer vaccine, which Moderna has been developing with Merck (MRK).
Needless to say, both hold the potential to become game-changers not only for Moderna but also for the entire industry.
Aside from its personalized cancer vaccine, another relatively advanced program in its pipeline is its work with AstraZeneca (AZN) on the AZD8601 program.
The AZD8601 program aims to use mRNA therapies to encode for vascular endothelial growth factor-A in people who are supposed to go through a coronary artery bypass grafting.
In layman’s terms, AstraZeneca and Moderna want to develop a treatment that induces the heart blood vessels of heart bypass surgery patients to repair themselves.
However, the most exciting collaboration is Moderna’s work with Vertex (VRTX) to develop a cystic fibrosis (CF) treatment.
Considering that Vertex is practically a monopoly in the CF space, this can turn out to be a lucrative direction for Moderna as well.
In terms of competition, the biotech might go head-to-head against Vertex’s other partner, CRISPR Therapeutics (CRSP).
Until two years ago, Moderna was an obscure biotechnology company with no product out on the market.
Today, it is hailed as one of the biggest biotechs worldwide thanks to its market capitalization of roughly $120 billion, surpassing long-established names in the sectors like Gilead Sciences (GILD) and even Vertex.
Some investors point out that Moderna’s breakneck rise to the top might also mean a steady descent.
While I agree that its climb was faster than the usual biotech, I still believe that Moderna possesses the right tools to sustain its momentum for the years to come.