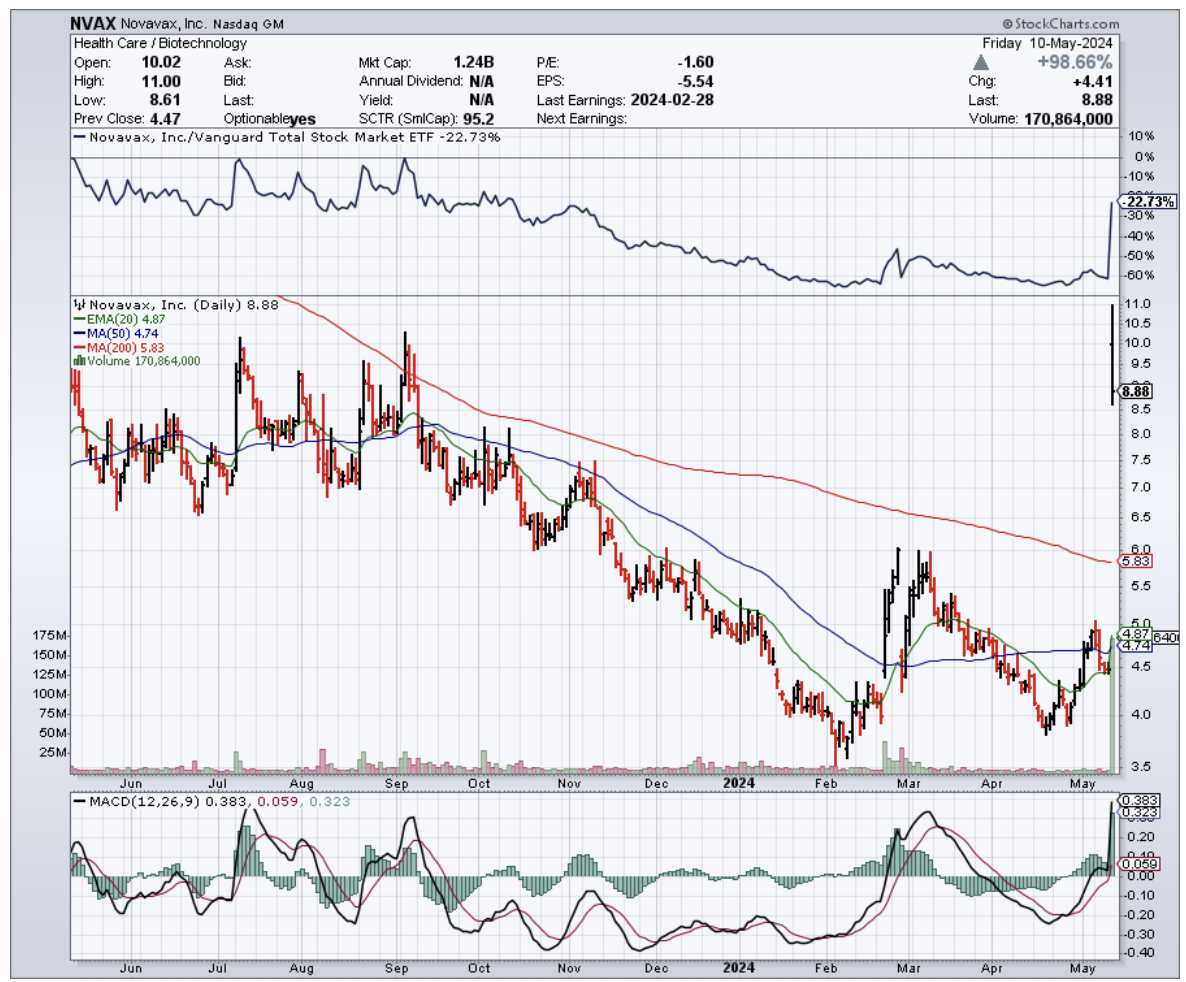
What a rollercoaster it’s been for Novavax (NVAX). Their latest Q1 2024 earnings were a mixed bag—they beat EPS expectations but missed on revenue. A far cry from when they were the undisputed rising star of the biotech world, their stock soaring a mind-boggling 6000% during the peak pandemic days.
Back then, they were the hotshots, but lately, the big leagues with the right connections had been sweeping opportunities right out from under them. Until now.
Earlier this week, Novavax’s shares took off like a rocket, even hitting a mind-blowing 175% jump in premarket trading at one point. That's right – 175%!
Turns out, those Q1 earnings weren't all doom and gloom. Sure, they missed on revenue, but their net loss got chopped in half compared to last year, and revenue still managed to grow by a respectable 16%. This isn't just a rebound; it's a sign that Novavax might just be back in the game.
But let's be real, the main reason behind this stock explosion is their shiny new deal with Sanofi (SNY), a heavyweight in the pharma world. We're talking a cool $1.2 billion.
Remember, Novavax was once the darling of the biotech scene during the pandemic, their stock soaring a mind-boggling 6000%. But lately, they've struggled to secure the big partnerships needed to really make a splash in the market. This Sanofi deal? It's the lifeline they've been waiting for.
This isn't just any partnership. Sanofi's shelling out a cool $500 million upfront, with another $700 million on the line if certain milestones are met, all for a piece of the Novavax COVID-19 vaccine pie and a chance to collaborate on future projects.
Now, Sanofi isn't exactly known for throwing money around willy-nilly, so this is a major vote of confidence. What's got them so excited?
Novavax's not-so-secret weapon: Matrix-M, a revolutionary adjuvant technology that's got the potential to shake up the vaccine world.
Think of Matrix-M as a personal trainer for your immune system. It doesn't fight the disease itself, but it whips your body's defenses into shape, making them stronger and more effective at fighting off infection.
To understand this better, imagine you're going into a boxing match. The vaccine is the boxer, ready to throw punches at the virus. But Matrix-M is the coach in their corner, giving them the extra training and conditioning they need to deliver a knockout blow.
Novavax isn't just using this personal trainer for their COVID vaccine. They're exploring how to use it to coach our immune systems in fighting all kinds of diseases, even the heavyweights like cancer. It's like having a secret weapon that could revolutionize how we approach health and wellness.
That means Matrix-M technology has the potential to open up a whole new world of treatment options and revenue streams. It's like investing in a gym that's developing a revolutionary new training program – the potential gains could be huge.
Now, this tech isn't a sure thing yet, but Sanofi's backing is a big deal. It's a vote of confidence that screams, "We believe in you, Novavax!"
And when a pharma giant like Sanofi puts their money where their mouth is, you know they see serious potential in those nanoparticle innovations and adjuvant magic. After all, who better to mass-produce a vaccine than one of the biggest players in the game?
This isn't just about COVID-19, either. This is about building a foundation for a whole new generation of vaccines, the kind that could rewrite the rules of healthcare as we know it.
Novavax isn't just sitting back and waiting, either. They're already gearing up for Phase III trials of a combo COVID-19-Influenza vaccine and diving headfirst into cutting-edge mucosal vaccine technology and high-density nanoparticles.
And let's not forget the cold, hard cash this deal brings to Novavax.
With half of Sanofi's investment expected to hit their bank account in just 10 days, Novavax's 2024 financial outlook is looking a lot brighter. They're now projecting revenues between $970 million and $1.17 billion – a serious boost for a company that's seen its share of financial turbulence.
Novavax might have been down for the count, but they're not out of the fight. With Sanofi backing them up, they've got a real shot at becoming a major player in vaccine innovation again.
For investors, this could be a chance to get in on the ground floor of a comeback story that could be the stuff of legend.