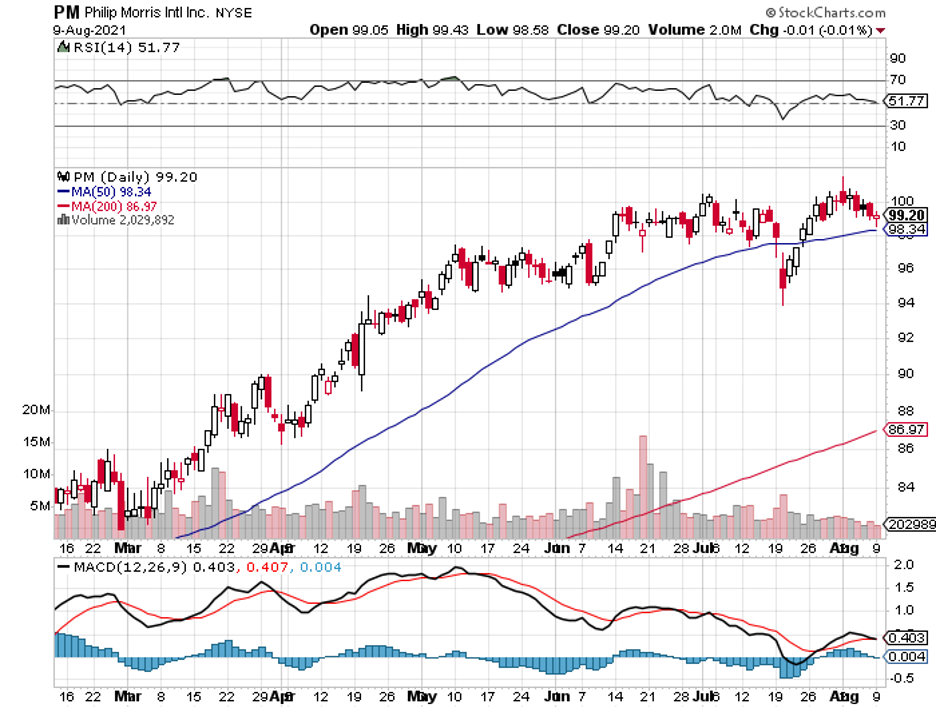
Philip Morris (PM) has long been synonymous with tobacco products, with the company dating all the way back in 1847.
Over 170 years later, though, this $154.6 billion company has gathered and read the tea leaves—and it looks like it finally realized that the future is “smoke-free.”
In a complete about-face, the Marlboro maker is now telling people that it wants to find a cure for heart and lung problems—the very same health conditions people get from tobacco.
Turning from poacher to gamekeeper, Philip Morris has decided to use its widely known expertise in inhalation to improve the health of the people.
One of its major moves towards this decision is to gatecrash the bidding for Vectura, a UK-based contract development and manufacturing company (CDMO) for the healthcare and pharmaceutical industry.
Vectura is known for its expertise and device technology in inhaled products, and the company has been working with numerous companies in the healthcare field, including GlaxoSmithKline (GSK), Novartis (NVS), and Bayer (BAYN).
Basically, Vectura develops treatments and medicines that help manage and cure smoking-related diseases.
So far, Vectura has 13 inhaled medicines along with 11 non-inhaled treatments in the market today.
To date, Philip Morris has raised its offer price to beat out the other contenders bidding for Vectura.
It’s now offering $2.30 per share, with the total reaching roughly $1.4 billion. That puts an almost 45% premium over Vectura’s shares—an offer that experts believe wouldn’t be matched elsewhere.
Considering that Vectura only generated $245 million last year, this is a massive gamble for Philip Morris.
This move comes after Philip Morris acquired Fertin Pharma for $820 million in July.
Fertin is known for developing nicotine chewing gum, lozenges, chewable tablets, and “pouch powders” that quickly dissolve in the user’s mouth.
Considering that Fertin only generated roughly $160 million in sales in 2020, Philip Morris’ move to buy it at that price point also speaks volumes of its plans moving forward.
Although it has yet to wrap up its Vectura acquisition, Philip Morris is not one to wait around.
While waiting for a decision on the British company, it snapped up another respiratory drug development company: OtiTopic.
Founded in 2012, OtiTopic’s main work is focused on Aspirhale, which is a late-stage inhalable acetylsalicylic acid for intermediate to high-risk acute myocardial infarction.
In simpler terms, OtiTopic is working on an inhaled aspirin for heart attacks.
Specifically, Aspirhale works as a dry powder inhalation via a self-administered aerosol.
This is promising since early research indicates that inhaled systems can alleviate the condition in two minutes compared to the 20 minutes needed for chewable aspirin.
With Philip Morris behind it, OtiTopic expects to complete testing soon and file for FDA approval by early 2022.
Considering the massive and expanding market for inhaled therapeutics science, the work of OtiTopic and Philip Morris is estimated to generate at least $1 billion in net revenues by 2025.
Moreover, the tobacco company anticipates the market for inhaled therapeutics will rapidly grow in the next few years and reach as much as $41 billion globally by 2026—a reasonable justification for its decision to splurge on Vectura as well.
When Philip Morris announced that it’ll stop selling cigarettes in the United Kingdom in 10 years, a lot of people thought it’ll be folding up shop.
Truth be told, the company’s about-face to healthcare wasn’t the strongest possibility entertained by the market.
Most believed that it would join forces again with its spinoff, Altria Group (MO), to form a stronger company in the industry and become a powerhouse of smoke-free alternatives.
However, its recent acquisitions indicate that Philip Morris plans to use Fertin, OtiTopic, and Vectura as the core of its legacy business in the healthcare and pharmaceutical industry.
According to Philip Morris, these decisions are part of its "natural evolution into a broader healthcare and wellness company."
While it does feel a bit like an arsonist marketing fire damage insurance, Philip Morris appears to be putting money where its mouth is in terms of transforming itself into a serious life sciences company.