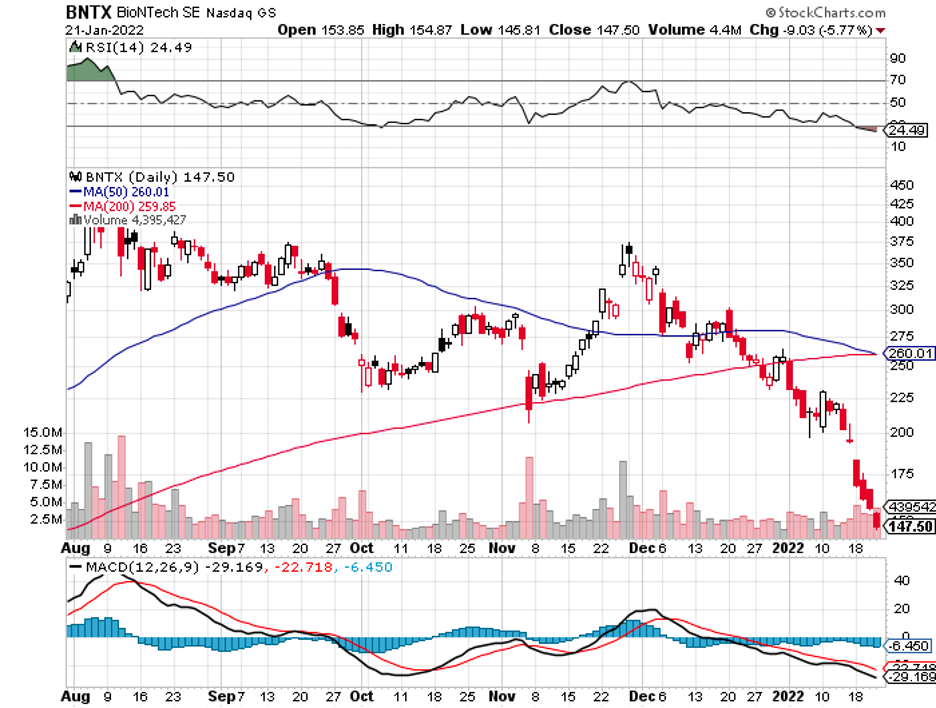
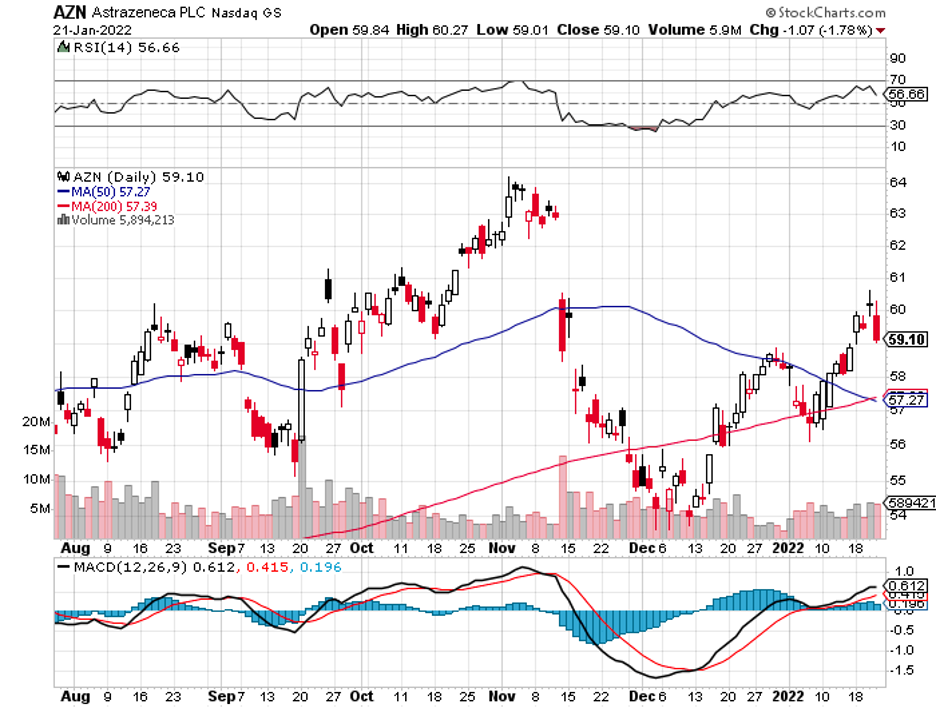
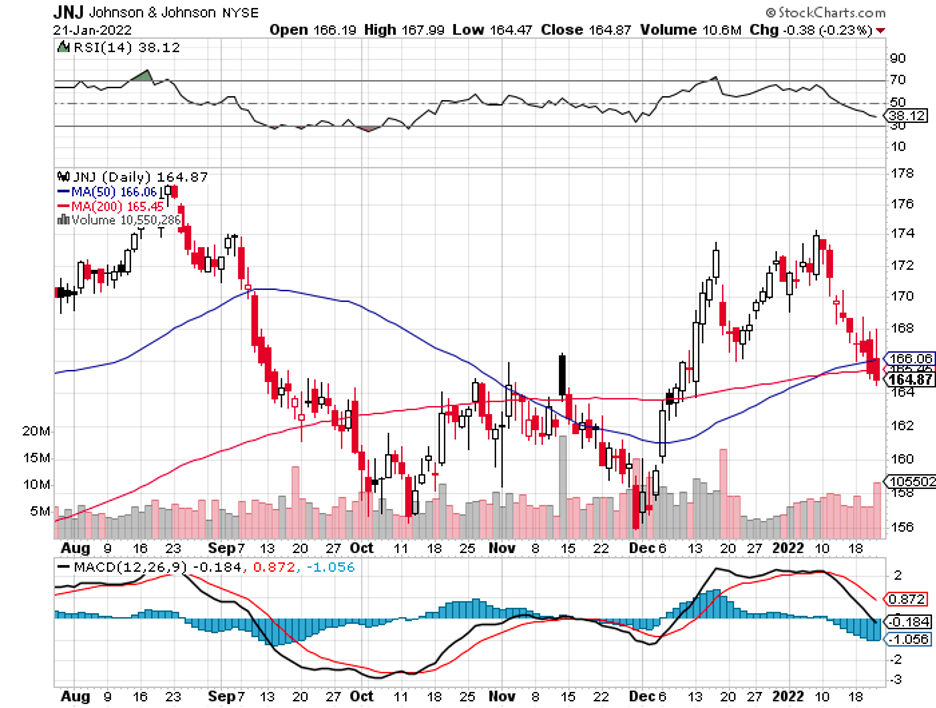
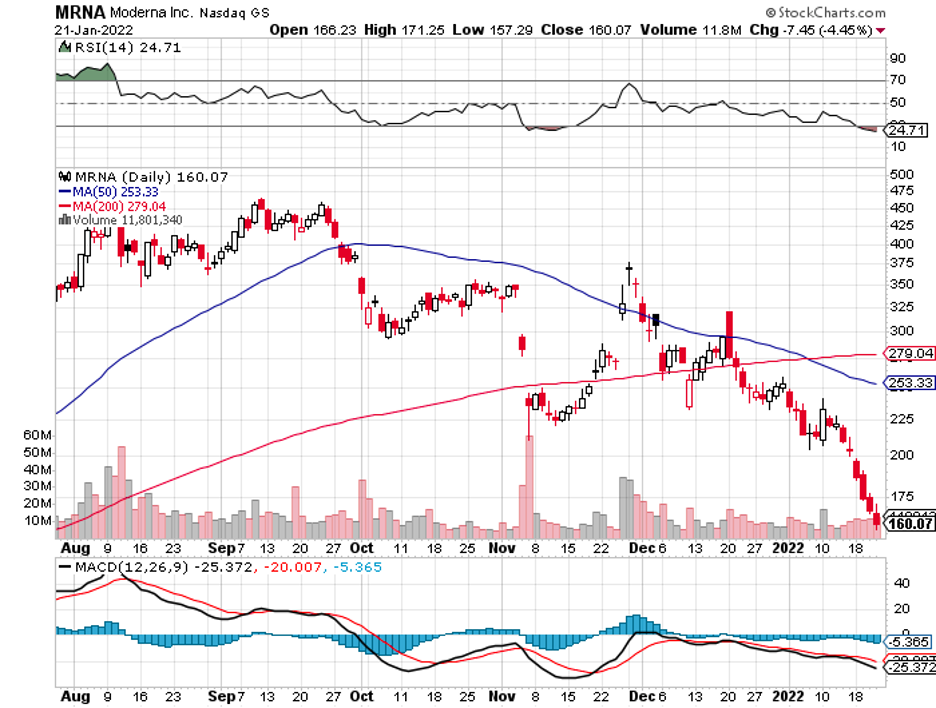
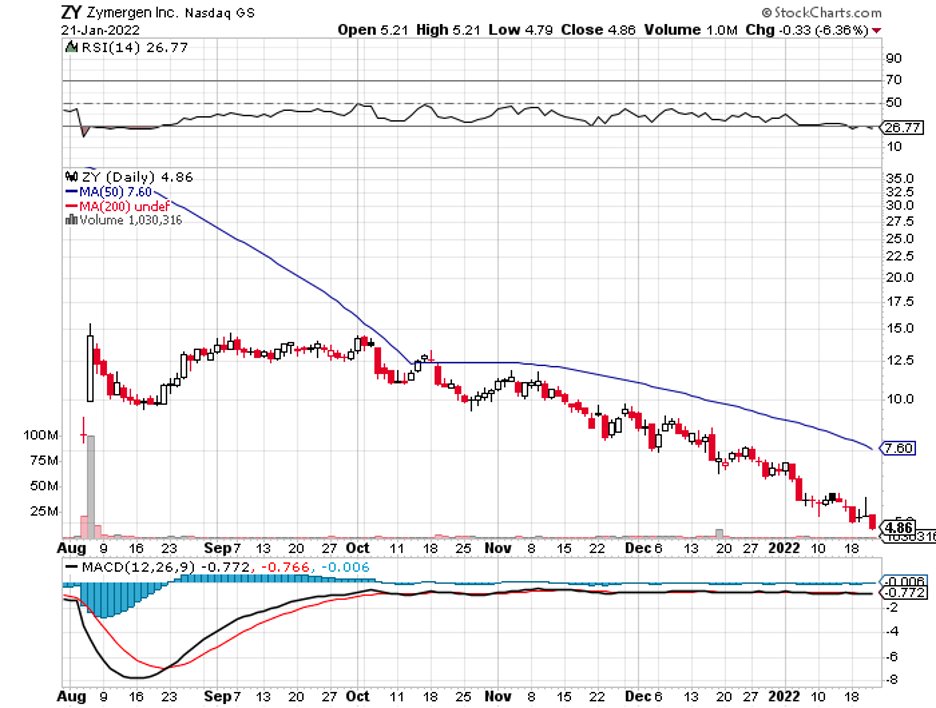
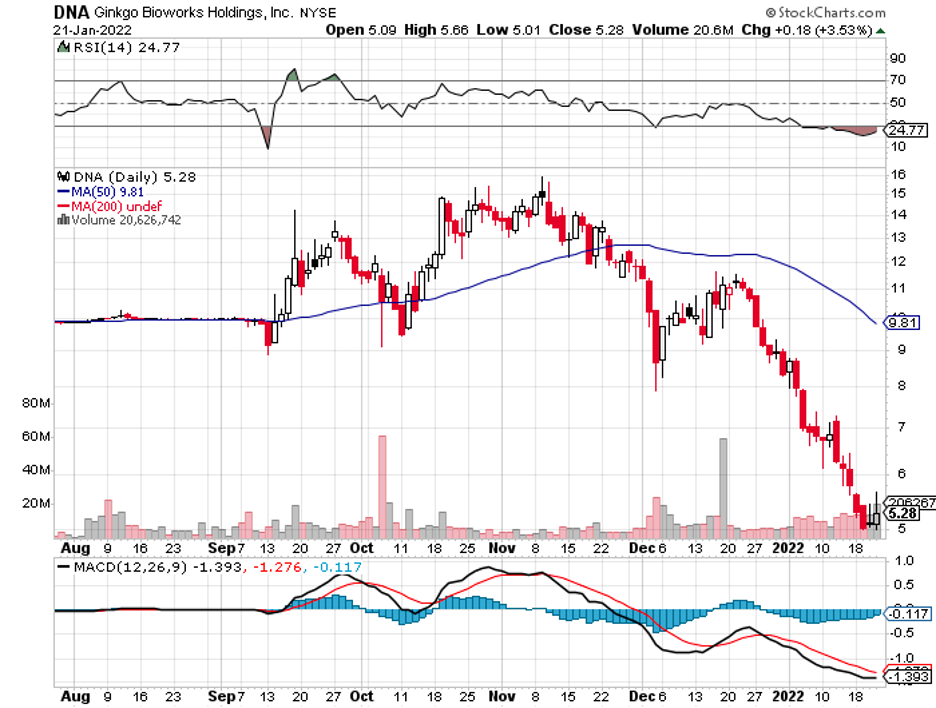
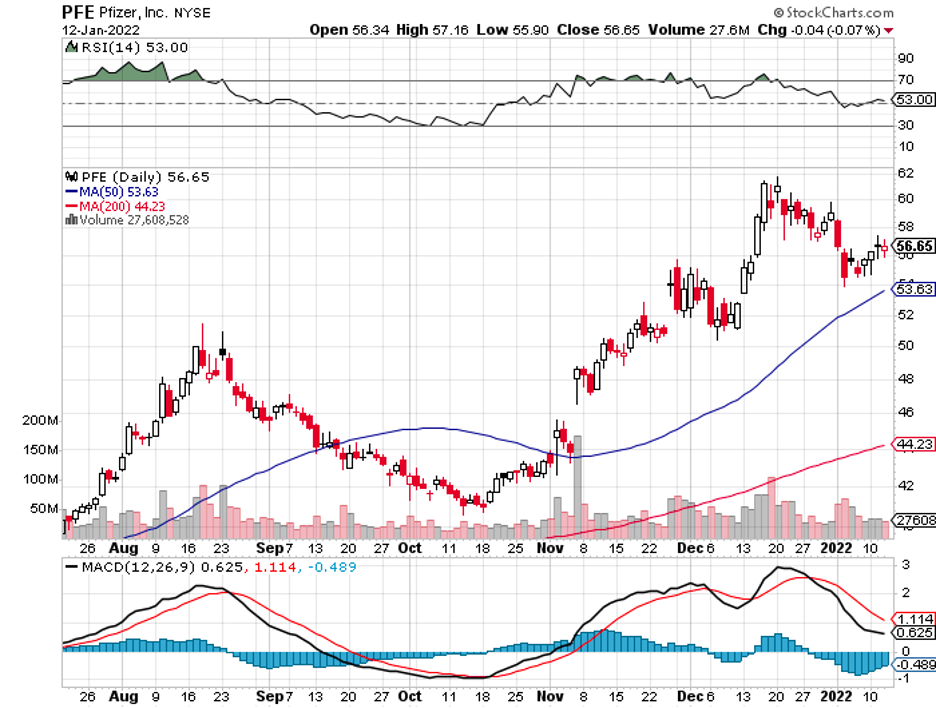
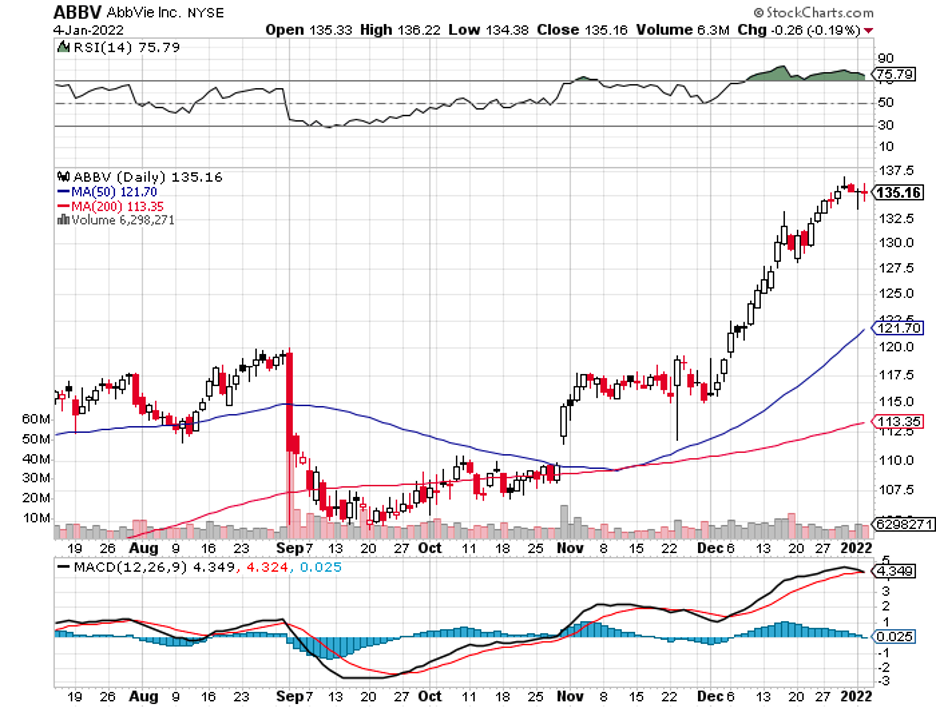
Industry experts typically describe mergers and acquisitions as the life force that propels the biotechnology and healthcare sector forward.
Based on that description, it’s safe to say that the segment’s health has plummeted, considering the sluggishness observed last year.
In 2021, the M&A of this industry had fallen to one of its lowest recorded levels in history.
During this period, the deals only amounted to $108 billion for the entire year. This number was approximately 40% of the total recorded in 2019.
Despite the sluggishness in 2021 and the relatively slow start in 2022, this year is still projected to push the would-be buyers into more aggressive action.
After all, several key products are facing patent expiration before this decade ends.
The list includes Big Pharma players like Pfizer (PFE), Bristol Myers Squibb (BMY), Merck (MRK), and Novartis (NVS).
This means that a massive deal might be on the horizon, pretty much when AbbVie (ABBV) executed its jaw-dropping $63 bill acquisition of Allergan in 2019 following its problems with generics competing against its blockbuster drug Humira.
Aside from patent protection concerns, another factor in play is the intense competition in lucrative research sectors such as immunology, neurology, rare diseases, and oncology.
Add to this the constant pressure of Congress to pull down drug prices, and it becomes apparent why companies—big or small—turn to mergers and acquisitions for survival.
Simply put, biotech and healthcare companies have no other choice but to be aggressive in looking for external innovation to secure the continuous transformation of their businesses.
On that note, I think there could be major acquisitions to be announced in 2022.
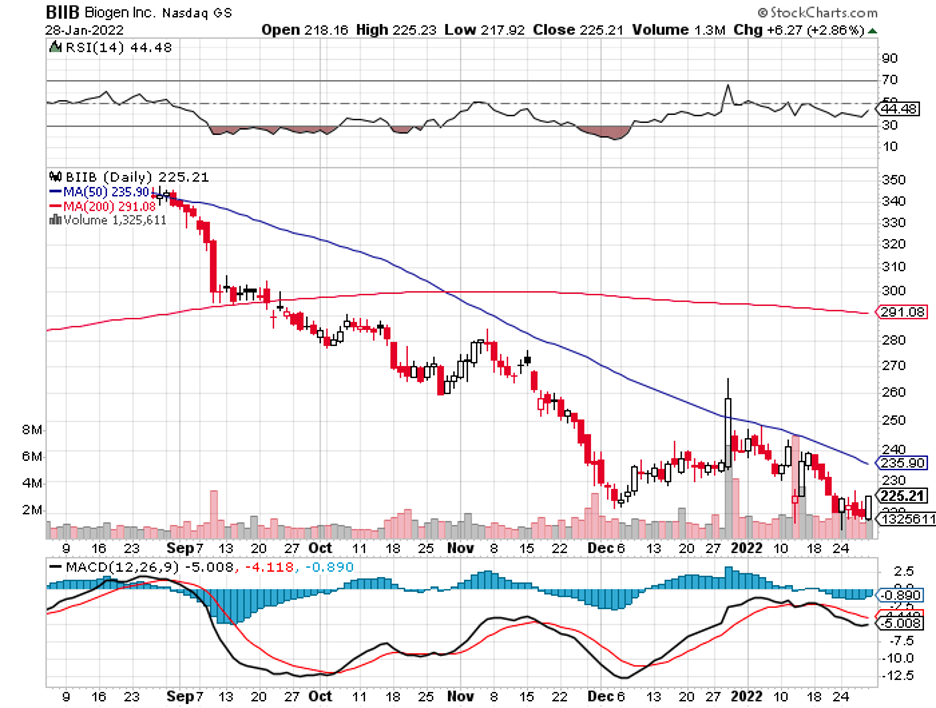
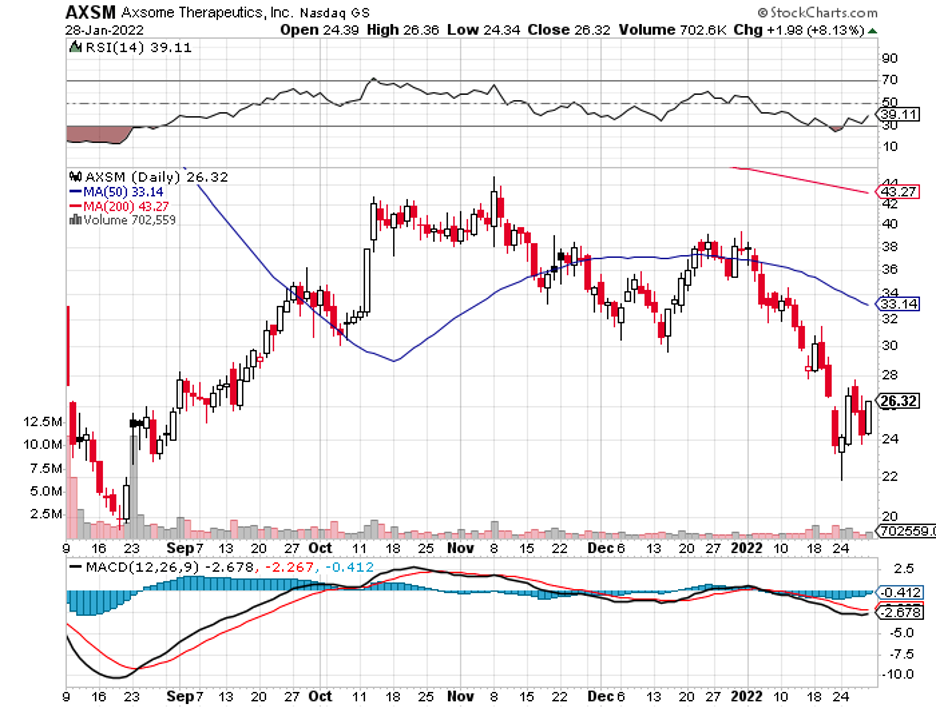
One deal I’m looking forward to is Biogen’s (BIIB) potential acquisition of Axsome Therapeutics (AXSM).
To remain competitive in the neuro stage, Biogen must keep up with the times—and a deal with Axsome might just be the solution.
Axsome’s size and price, with a market capitalization of $992 million, appear to be just the right fit for Biogen to gobble up.
More importantly, its portfolio is an excellent fit for Biogen. Both focus on neurological diseases, making their pipelines complementary to each other.
So far, Axsome has several leading candidates in the clinical stages.
One is AXS-05, which is a treatment for major depressive disorder (MDD).
Apart from MDD, this candidate is under late-stage review to target Alzheimer’s disease agitation.
In addition, Axsome is looking to advance AXS-05 in late-stage trials for smoking cessation therapy.
Needless to say, AXS-05 would go hand in hand with Biogen’s own approved, albeit controversial, Alzheimer’s drug Aduhelm.
Another promising candidate is AXS-07, a potential competitor of Pfizer and Novartis’ migraine medication. This drug has been submitted for FDA approval and might be launched by the second quarter of 2022.
There’s also AXS-12, which is a narcolepsy treatment candidate, and AXS-14, which is geared towards fibromyalgia. Both candidates are slated for FDA review by the third or fourth quarter of 2022.
For over 20 years, even the biggest and most powerful drug companies have stayed away from working on treatments specifically for the brain and central nervous system (CNS).
That’s not surprising considering the sheer number of failed programs in neuroscience, pushing drugmakers to believe that we still don’t have sufficient data on the subject, so the money might be better spent elsewhere.
Nowadays, though, the CNS landscape is starting to shift.
GlaxoSmithKline (GSK) recently embarked on reviving its CNS program by striking a $700 million deal with a smaller biotechnology company called Alector.
Meanwhile, Pfizer and Novartis reached an agreement with Biohaven Pharmaceuticals for the latter’s migraine treatment and Parkinson’s drug.
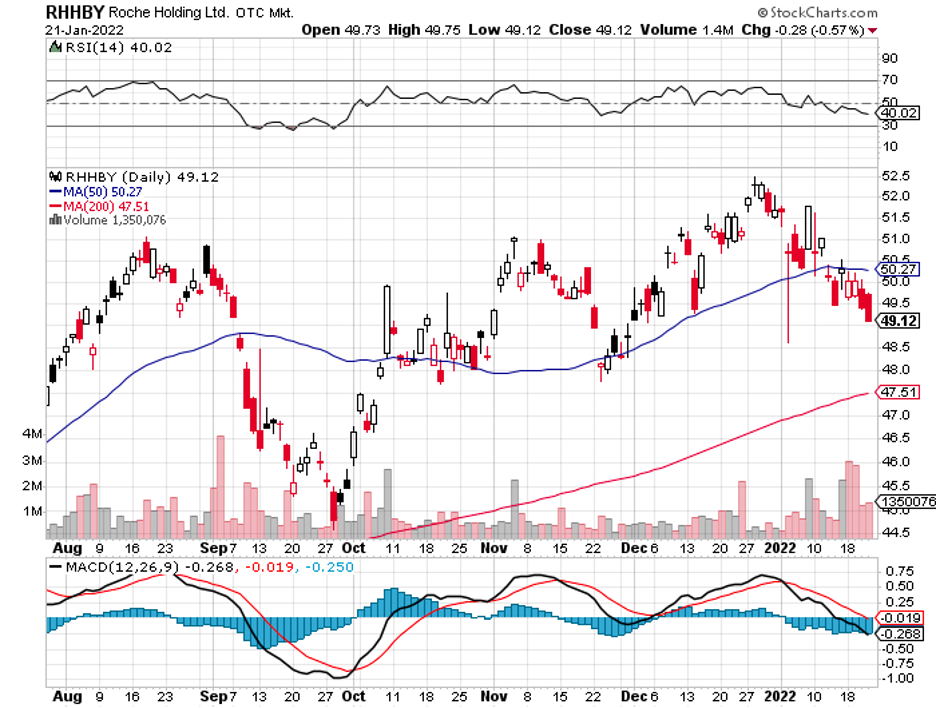
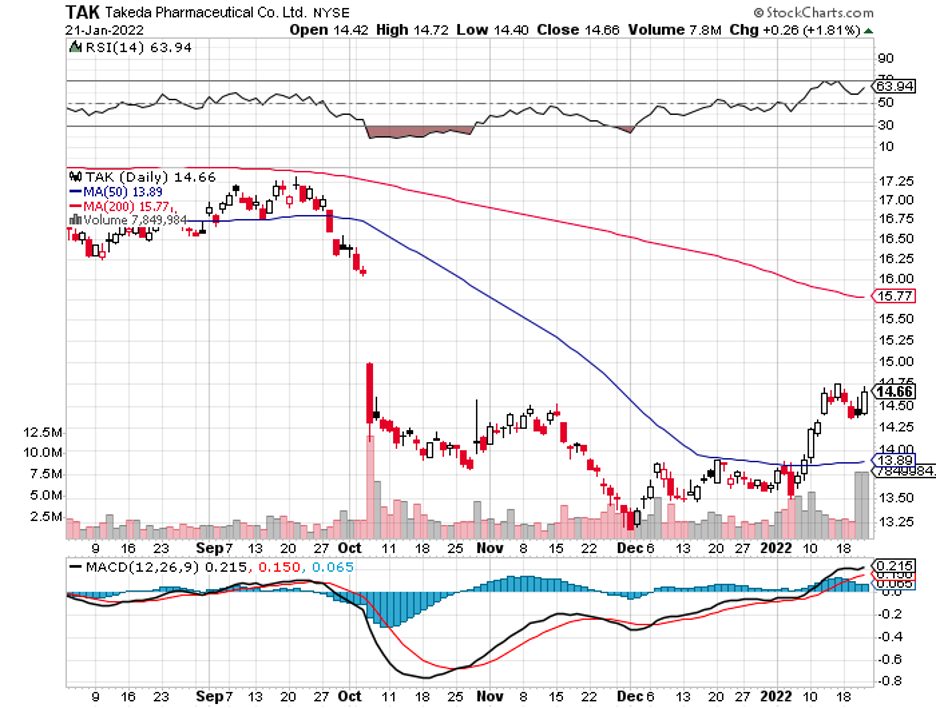
Aside from these, Johnson & Johnson (JNJ), Eli Lilly (LLY), Roche (RHHBY), and Takeda (TAK) are anticipated to secure CNS-centered deals soon.
Despite the lower number of M&A deals last year, the volume of strategic collaborations in the neuroscience sector climbed by about 50% in 2021 compared to its 2020 performance.
By 2022, this space is projected to become even more investable, considering the number of biotechnology companies focusing on CNS. Watch out for blockbuster deals in this sector.