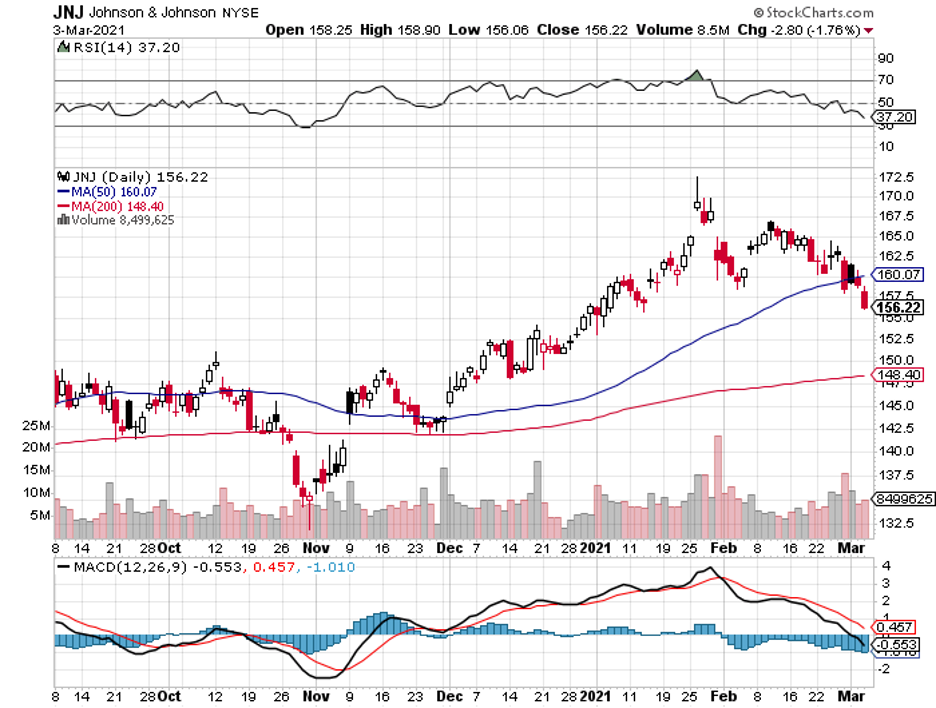
The past few weeks have been hectic for the healthcare industry, with Moderna (MRNA), Pfizer (PFE), BioNTech (BNTX), and even Johnson & Johnson (JNJ) working hard to manufacture and distribute their COVID-19 vaccines all over the world.
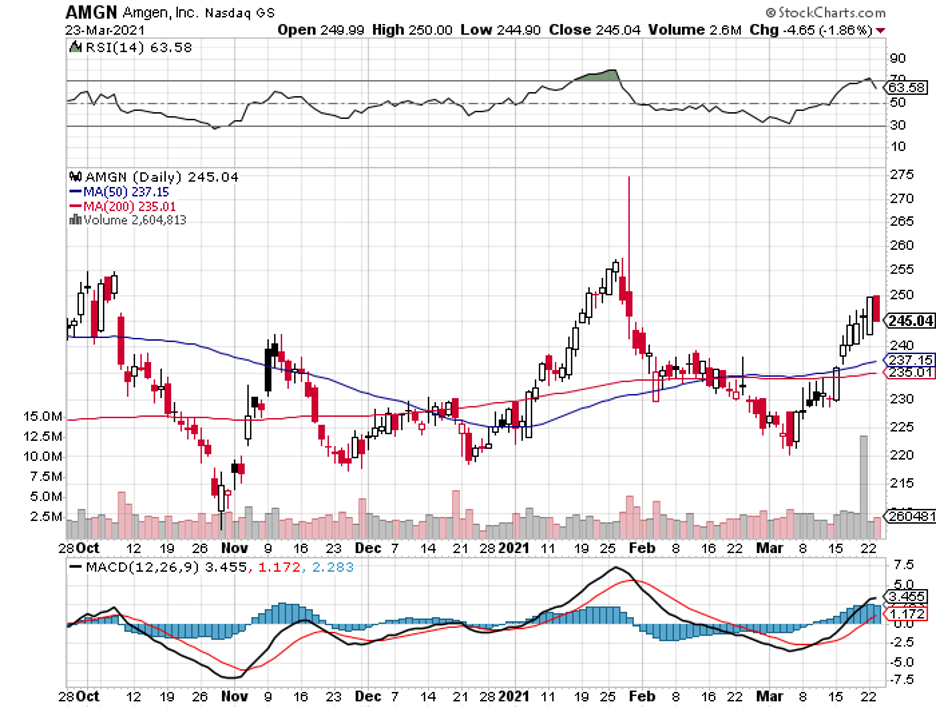
There’s one major player in the healthcare industry that has been out of the spotlight for quite some time: Amgen (AMGN).
While Amgen has been doing its part from the sidelines by helping out companies like Eli Lilly (LLY) with the manufacturing of their COVID-19 drugs, it looks like investors are flocking towards businesses that allocate more resources toward fighting off the pandemic.
In fact, JNJ recently reached a new high at $170 per share.
Nonetheless, I think investors are missing out on a great opportunity by ignoring Amgen these days.
The biotech world, which basically involves formulating drugs and treatments for living organisms, was somewhat limited back in 1980.
Over the past decades, however, this industry has shifted and managed to successfully launch groundbreaking drugs commercially.
Before, only a handful of legacy companies had occupied this space. Now, so many up-and-coming companies try to conquer the biotech world.
For context, an FDA report in 2019 showed that 64% of drugs approved in the previous year were developed by biotech companies.
Moving forward, it’s reasonable to say that the biotech industry will continue to come up with breakthrough treatments for rare and complex conditions compared to our traditional pharmaceutical companies.
Actually, this sector has been hot in recent years, with companies like AbbVie (ABBV) and Celgene, now with Bristol-Myers Squibb (BMY), coming up with mega-blockbuster treatments, such as Humira and Revlimid, that rake in billions in sales annually.
Among them, Amgen has emerged as one of the most consistent and aggressive players in the biotech world, with competitors still struggling to topple some of its products after decades of being in the market.
This biotech giant has also been busy boosting its pipeline of newly developed treatments. It’s even bolstering its biosimilar lineup to ensure its dominance in the sector.
Last year, Amgen’s revenue rose by 9%, with more growth indicators lighting the way for a brighter future for the company.
While Amgen has been working on many conditions, its portfolio still looks focused on particular diseases.
In 2020 alone, Amgen’s seven blockbusters each generated over $1 billion in revenue. Among these, four managed to rake in more than $2 billion in annual sales.
Amgen’s impressive lineup of drugs includes psoriatic arthritis treatment Enbrel, osteoporosis and bone cancer injection Prolia, and even newcomer heart disease medication Repatha.
With its rivals nipping at the heels of its first-generation blockbusters like Neupogen, Amgen has been hustling to find ways to reinvent itself.
Apart from developing new drugs, the company has been looking into acquisitions to sustain its position at the top.
Recently, Amgen has been doubling down on its newest shining star: Otezla.
Otezla was one of the company’s biggest purchases, with Amgen acquiring this drug for a whopping $13.4 billion from Celgene in August 2019.
In 2019, Otezla sales rose by 25% to reach $1.6 billion. By 2020, the drug generated $2.2 billion in sales, showing off a 36.5% jump.
Over the next few years, Amgen estimates that Otezla sales will climb by over 10% annually.
Riding the momentum of not only Otezla but its entire portfolio and programs in the pipeline, Amgen aims to dominate the immunology sector.
Among the candidates in Amgen’s pipeline, the most promising is its lung cancer medication Sotorasib, which should complete Phase 2 in the first half of 2021.
Meanwhile, Amgen’s latest deal outside its own pipeline is the $1.9 billion acquisition of Five Prime Therapeutics (FPRX), which is a small biotech company developing treatments for stomach cancers. The agreement should be finalized by June 2021.
Five Prime’s experimental treatment, Bemarituzumab, perfectly aligns with the other stomach cancer medications queued in Amgen’s pipeline.
If this proves successful, then Bemarituzumab will be a strong contender against Bristol-Myers Squibb’s blockbuster treatment Opdivo.
While Opdivo has been in the market longer, Five Prime’s candidate has consistently shown stronger and more promising results since the trials started.
Prior to its deal with Prime Five, Amgen acquired a 20% stake in Beijing-based biotech company BeiGene (BGNE). This is a telling move as it indicates the company’s efforts to expand its reach in Asia, particularly in China and Japan.
Another revenue stream that Amgen has been pushing for expansion is its biosimilars sector.
The company released its first-ever blockbuster, Epogen, in 1989. Since then, this anemia drug has been a top seller. However, biosimilar competition eventually caused a decline in its sales starting in 2015.
Learning from the fall of Epogen in the hands of biosimilars, Amgen decided to turn its weakness into its strength.
Since 2015, the company has been expanding its work on biosimilars. In that year alone, Amgen developed 29 biosimilars for its own products and launched 18 more to compete with other companies.
To date, biosimilars have been generating at least $2 billion in revenues, with 10 more queued in Amgen’s pipeline.
Considering the accelerated growth of the biotechnology sector, now is not the time to count out Amgen.
Today, Amgen has transformed itself into one of the leaders in the biotech world, generating over $25 billion in revenue.
Since 1988, the company has only reported a decline in its year-over-year revenue three times: 2009, 2018, and 2019.
This performance shows tangible proof that Amgen is not a “one-hit-wonder” type of biotech stock. Instead, it demonstrates its capacity to generate solid earnings and sustainability.
Currently, Amgen trades at a price-to-earnings multiple that’s actually 40% lower than the average S&P 500 stock. Its EPS is estimated to rise in the high single digits in the next several years.
Simply looking at its 2020 fiscal report, it’s obvious that Amgen delivered an impressive performance considering the recession and the pandemic.
The company also continues to reward its shareholders with double dividend increases plus an aggressive repurchase program, which Amgen plans to spend roughly $3 billion to $4 billion.
Recently, the stock has been trading at a roughly 30% discount. This is a real bargain considering everything Amgen has to offer.