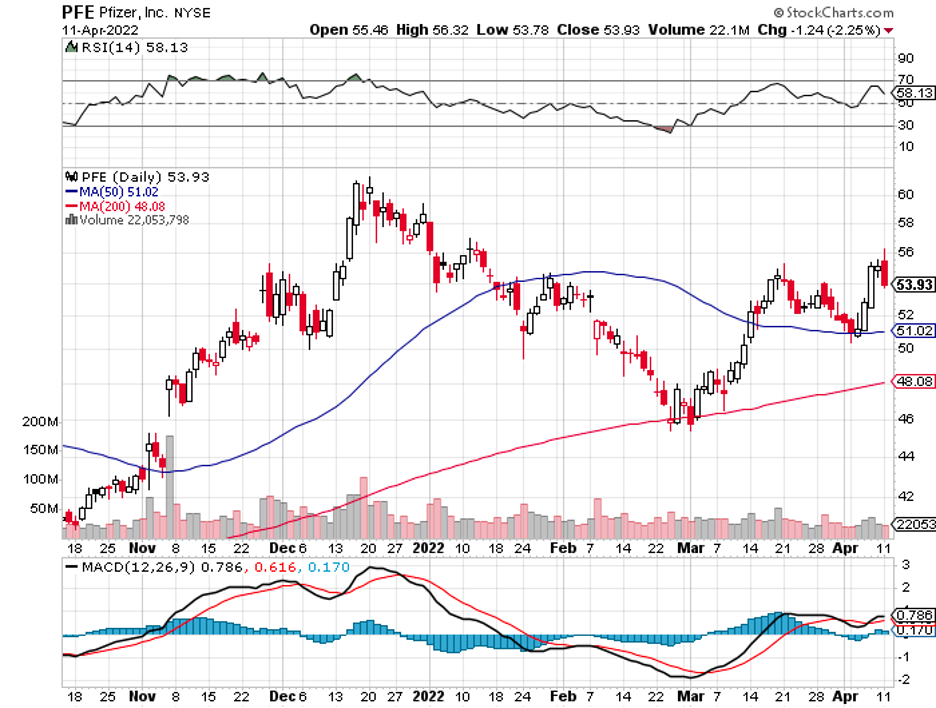
Being one of the most famous names in the biotechnology and healthcare industry, Pfizer (PFE) barely needs any introduction.
Thanks primarily to its COVID-19 vaccine, Comirnaty, Pfizer’s top line has skyrocketed, with its trailing 12-month revenue exploding by over 99% in the past three years and recording a total of a whopping $81.2 billion.
Realistically speaking, it’s best not to rate the chances of this happening again in the years to come.
Still, there are several factors that make Pfizer a convincing stock to hold even when the pandemic shifts to an endemic.
First, the COVID-19 program will most likely continue to rake in blockbuster revenues. Although it won’t be as high as the previous years, Comirnaty sales are projected to reach $39 billion this 2022 and $22 billion in 2023.
Apart from this vaccine, which was developed with BioNTech (BNTX), Pfizer also recently received approval for its own COVID-19 oral treatment called Paxlovid. The addition of this pill in its portfolio all but guarantees another high-growth revenue stream for the company.
Second, Pfizer holds eight blockbuster treatments focused on diverse sectors.
While these will eventually struggle with generic competition by 2030, the company has that issue covered. To date, Pfizer has roughly 89 candidates in its pipeline with 27 undergoing Phase 3 trials.
Pfizer’s plans to expand its pipeline became particularly evident in the past week as the company made some noise in the muted M&A scene.
Right on the heels of its successes in its lead RSV vaccine candidate, Pfizer bolstered this program through a $525 million biotechnology buyout.
The company that caught this Big Pharma’s attention is ReViral, which has been hard at work in developing Sisunatovir, an oral RSV drug.
While eyebrows may have raised over the price tag for a company with a single asset, it should be noted that Sisunatovir is estimated to rake in $1.5 billion in annual sales—and this pill isn’t the only candidate in ReViral’s pipeline.
All in all, that’s obviously not a bad payback for a contract this size.
The RSV space has always been a challenging and lucrative market for biopharmas, with the global costs linked to this disease reaching $5.45 billion in 2017.
Researchers have been working on a vaccine for decades, with some experiments dating as far back as the 1960s.
With the addition of ReViral to its portfolio, Pfizer has clearly positioned itself as the frontrunner in the RSV vaccine race.
This puts it firmly ahead of GlaxoSmithKline (GSK), which had to suspend its trials for safety reasons, and even the partnership between Sanofi (SNY) and AstraZeneca (AZN).
Evidently, Pfizer’s deep pockets could indicate additional acquisitions of smaller companies with promising candidates in their pipelines.
We’ve seen this happen with ReViral and, prior to this, Arena Pharmaceuticals to the tune of $6.7 billion primarily for the smaller biotech’s encouraging anti-inflammatory treatment Etrasimod.
Although that price tag initially raised doubts about Pfizer’s spending, a deeper analysis of Arena’s pipeline showed that it could bring $28 billion per annum by 2025.
Flush with the billions it earned from its COVID-19 program, the recent ReViral deal appears to be a relatively minor one for Pfizer.
This leads me to believe that this move marks the beginning of a fresh season of biotech buyouts—and Pfizer might very well be in the lead.
Overall, Pfizer presents a compelling investment case. It is remarkably diversified, which means it offers below-average risks.
Considering its trajectory and putting it against the backdrop of the fast-growing biotechnology industry, Pfizer has the potential to become a trillion-dollar company within 20 years.