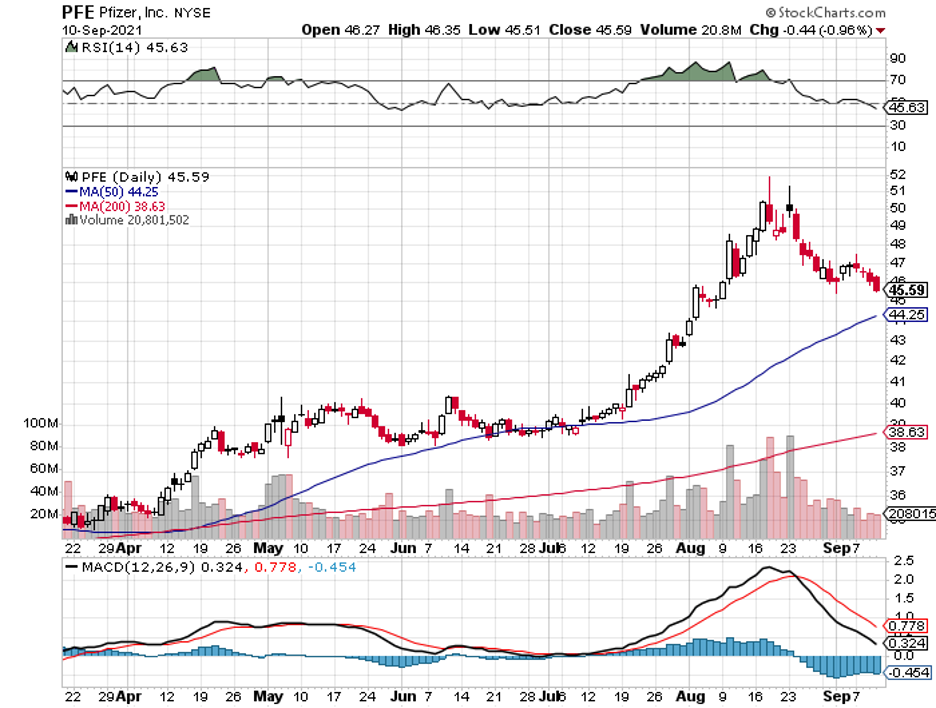
The pandemic is exhibiting hints of easing, and one of the names playing a critical role in the vaccine rollout that has made this step towards normalcy possible is Pfizer (PFE).
Actually, Pfizer stock has hit an all-time high courtesy of its COVID-19 vaccine, Comirnaty, which it developed with German biotech firm BioNTech (BNTX).
While this is undoubtedly an exciting time for the company, many investors wonder whether this period also marks the spectacular of Pfizer, and things will go downhill from here. After all, several of its patents are set to expire starting in 2025.
My short answer to this question is no. This isn’t the beginning of the end for Pfizer. Looking at the company’s history, pipeline, and trajectory, I can say that Pfizer’s rise is just getting started.
One of the key reasons behind my belief is Pfizer’s robust pipeline.
To date, the company has roughly 100 drugs queued for regulatory clearance, while others are slated for late-stage clinical testing.
That means that regardless of the patent expirations in Pfizer’s horizon, the company’s strong and diverse pipeline can easily counteract the blow from the loss of exclusivity.
Just last month, Pfizer received full approval from the US FDA for Comirnaty.
Since fellow vaccine developers like Moderna (MRNA) have yet to achieve the same, this makes Pfizer the first COVID-19 vaccine to gain this endorsement from the regulatory committee.
Needless to say, Pfizer could capitalize on this massive opportunity to boost its profits in the quarters.
The availability of a fully approved COVID-19 vaccine could allow establishments to oblige mandatory vaccinations, which could obviously lead to higher demand for Comirnaty, as over 100 million Americans have yet to receive at least a single jab.
In the second quarter of 2021, the company reported $19 billion in revenue, indicating a 92% year-over-year climb thanks to the $7.8 billion raked in by its COVID-19 vaccine.
Pfizer now estimates Comirnaty revenue to reach roughly $33.5 billion, indicating an expected 2.1 billion doses to be delivered within the year.
Excluding Comirnaty’s sales, Pfizer’s revenue increased by 10%. This strong momentum led the company to raise its 2021 full-year guidance to somewhere between $78 and $80 billion.
Before Comirnaty, though, Pfizer had already been known as a prolific vaccine developer.
One of its prized creations is the pneumococcal vaccine Prevnar, which generated $2.52 billion in revenue in the first 6 months of 2021.
Meanwhile, its tick-borne encephalitis vaccine, marketed as TicoVac, gained FDA approval in July and could bring in roughly $1 billion per annum.
Riding this momentum, Pfizer has been busy developing another potential moneymaker in this segment in the form of its respiratory syncytial virus (RSV) vaccine candidate: RSVpreF.
And if the Phase 3 results for RSVpreF come anywhere near its Phase 2 trial, then Pfizer has another blockbuster in its hands.
This is because the RSV vaccine market is projected to grow to approximately $10 billion by 2030, and Pfizer’s candidate is targeting 72% of that population.
However, the RSV market will be a crowded space with the likes of Johnson & Johnson (JNJ), Sanofi (SNY), and GlaxoSmithKline (GSK) working to fill this unmet medical demand.
So, realistically, Pfizer’s RSVpreF has the potential to capture 20% market share, translating to $2.1 billion in annual revenue.
Apart from its vaccine-related efforts, Pfizer’s core businesses have been growing as well. Top contributors come from its oncology arm, specifically Eliquis and Ibrance.
Its recent acquisition of Trillium Therapeutics (TRIL) is anticipated to serve as a catalyst for Pfizer’s cancer segment in the next years as well.
Overall, Pfizer has a blockbuster drug pipeline and an impressively successful COVID-19 vaccine rollout. These provide the company with a long runway for solid and steady growth.