The ideal stocks are those you can just buy and hold for a long time. A healthcare and biotechnology company that perfectly fits the bill is Amgen (AMGN).
Amgen wasn’t an active participant in the COVID-19 race.
Instead, the biotechnology giant chose to stick with its circle of competence and focused on delivering remarkable results to its shareholders through boosting its revenue and increasing dividends.
Recently, this hyper-focus has paid off.
Amgen received FDA approval to market a drug that targets cancer cells in an area that researchers have been attempting to hit for decades.
The new treatment, Lumakras, will be the first of its kind to target a tumor growth process commonly known as KRAS for non-small cell lung cancer (NSCLC).
To understand the extent of Amgen’s breakthrough, scientists and researchers have been working on developing a KRAS blocker for over 40 years.
Prior to this, KRAS had been known as an “undruggable” target.
Basically, Amgen came up with a drug that can target the notorious and illusive cancer-causing protein—something that was previously considered the “Achilles heel” of lung cancer tumors.
More impressively, Lumakras was approved three months ahead of its schedule.
Based on the results of its Phase 2 trials, Lumakras can stall the progress of lung cancer in roughly 81% of the patients for a median time of 10 months.
In the Phase 3 trials, Amgen is looking into testing the drug in combination with other medications to hit the tumors that developed resistance to the pill.
A key factor in Lumakras’ launch is determining the types of patients who’d benefit most from the drug.
So far, Amgen has been collaborating with diagnostic partners, particularly Qiagen (QGEN) and Guardant Health (GH), for biomarker testing.
In terms of pricing, Amgen estimates monthly spending on Lumakras to be $17,900.
In the United States, roughly 30,000 patients of KRAS-mutated lung cancers are reported annually.
That puts Lumakras sales to at least $100 million for 2021 alone.
By 2025, the drug is expected to rake in roughly $1 billion annually, with sales growing to $1.51 billion in 2026.
These are actually conservative estimates that assume only a 50% success rate from Lumakras in the next few years.
Given the provisional and accelerated approval the drug has already received from the FDA though, it is safe to say that it can achieve at least 75% success rate, which means it can generate higher revenue.
The KRAS target is not limited to lung cancer. It also appears in other solid tumors, which Amgen continues to test Lumakras in a dozen other types, including colorectal cancer.
Depending on expansion plans, Lumakras sales can reach $3.2 billion by 2030.
Again, this expansion is a conservative estimate.
If the expansion for Amgen’s drug would be anything like AstraZeneca’s (AZN) blockbuster Tagrisso, which eventually became a recommended first-line therapy option for NSCLC, then Lumakras sales can peak at $4 to $5 billion.
Considering the potential of this market, it no longer comes as a surprise that competitors are hot on Amgen’s heels just days after Lumakras’ approval was announced.
The closest rival so far is Mirati Therapeutics (MRTX), which also has KRAS-inhibitor candidates in Phase 1 and Phase 2 trials.
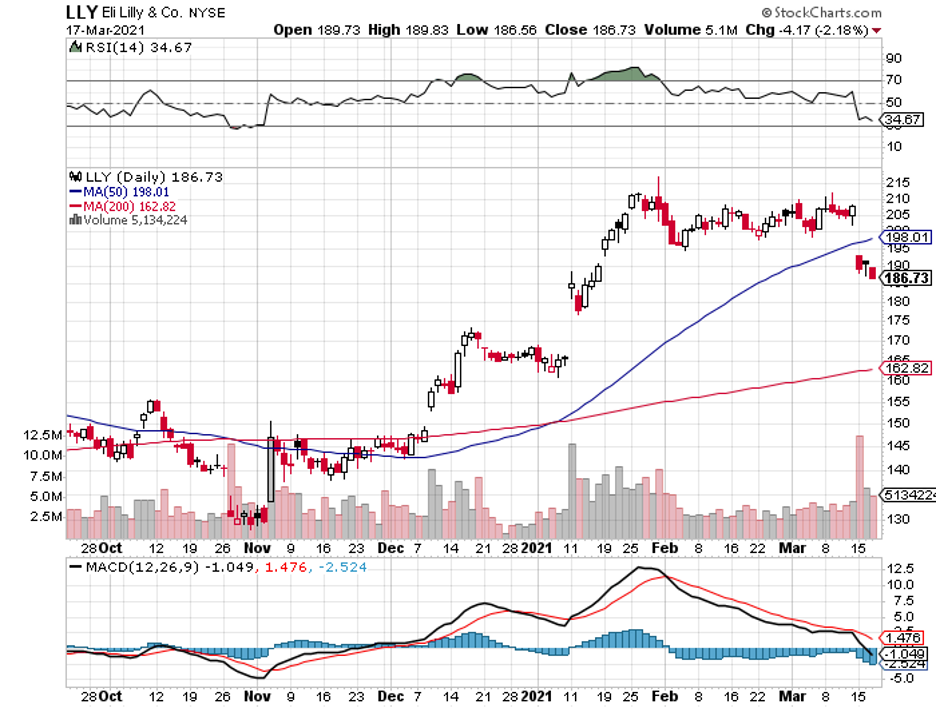
Prior to that, Eli Lilly (LLY) and Johnson & Johnson (JNJ) tried their hands at KRAS mutation but failed.
Aside from Lumakras, Amgen has another blockbuster candidate in store for its shareholders: asthma drug Tezepelumab.
Developed in collaboration with AstraZeneca, this drug is already in the second late-stage pipeline and has been showing promising results so far.
Globally, there are about 2.5 million patients with severe asthma, with 1 million suffering from eosinophilic asthma in the United States. Amgen is hoping to target the latter population.
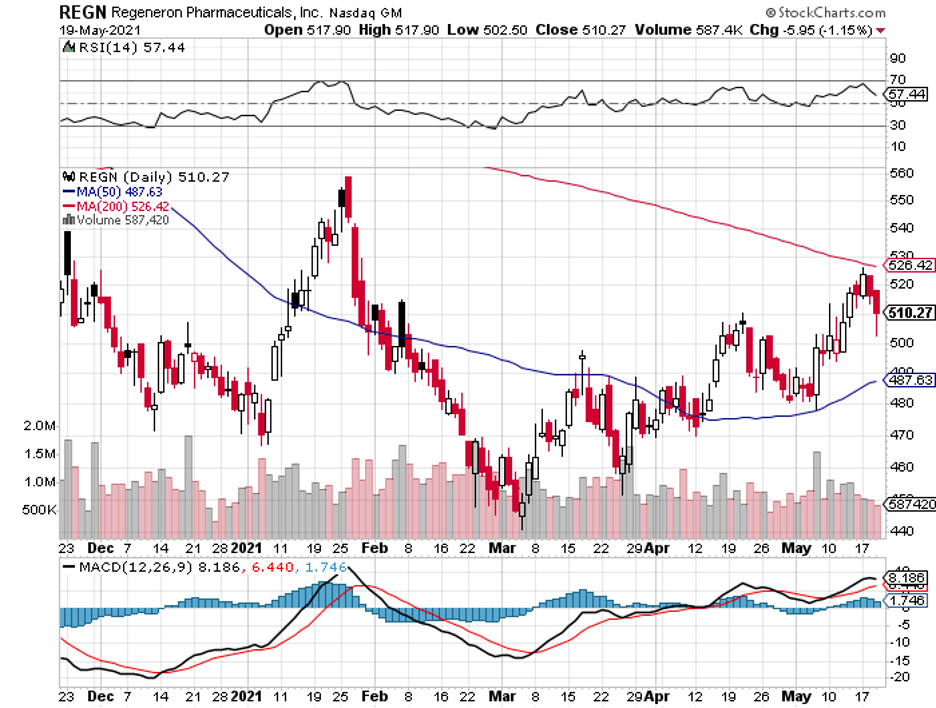
If Tezepelumab gets approved, it would be in direct competition against Sanofi (SNY) and Regeneron’s (REGN) asthma drug Dupixent. Peak sales for this asthma drug is estimated at roughly $3.5 billion.
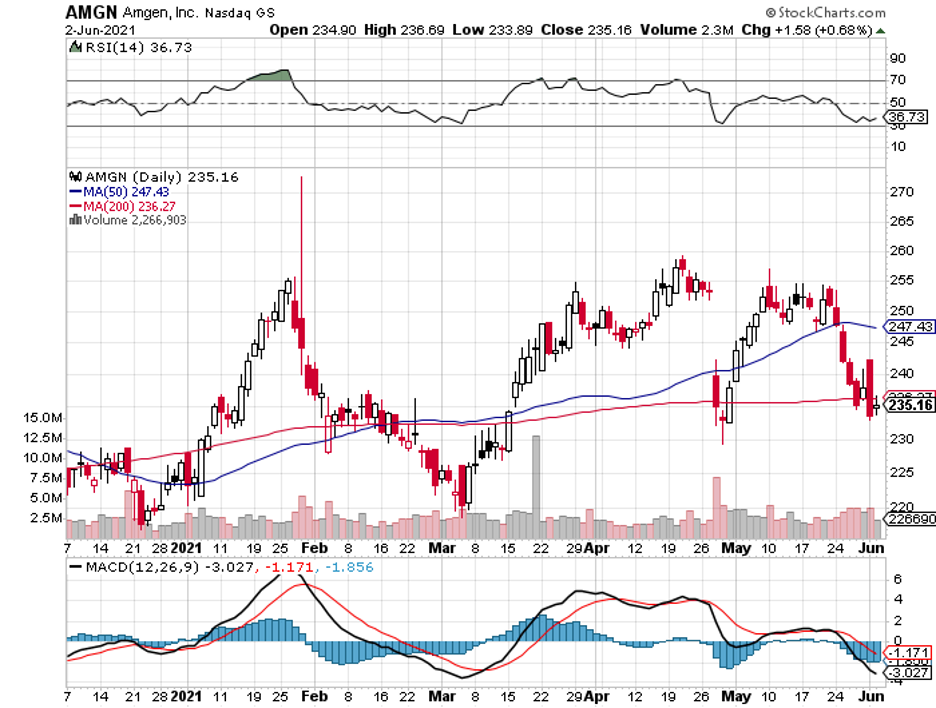
Over the past 12 months, Amgen’s stock performance has been rangebound.
Although this is obviously frustrating for growth-oriented shareholders, I think the short-term volatility of the stock may present good opportunities for value-conscious investors.
That is, I view the drop in Amgen’s share price as another favorable buying opportunity.