The Eyes Have It
Last week, while waiting for my annual eye exam, I couldn't help but notice the parade of elderly patients shuffling in for their regular Eylea injections. My optometrist tells me these folks show up like clockwork every 4-8 weeks, rain or shine.
That's about to change, and therein lies a multibillion-dollar story.
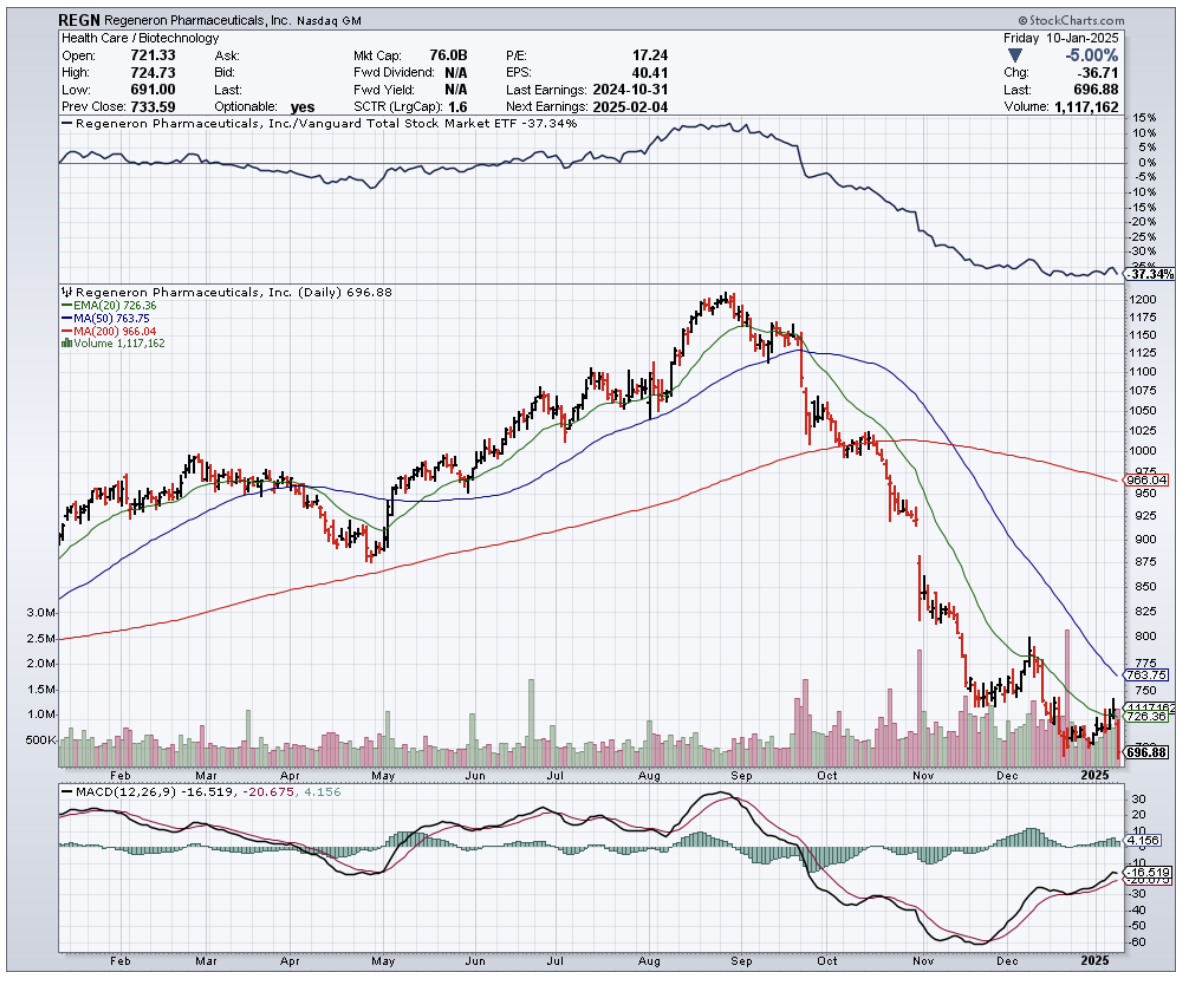
You see, when Regeneron reported Q3 earnings on Halloween, boy, they sure had some treats for investors. Revenue hit $3.72 billion, up 11% YoY, with EPS coming in at a sweet $11.54.
But here's what really caught my attention: their cost of revenue was $1.762 billion, while R&D and SG&A expenses ran $1.271 billion and $714.4 million respectively.
Net income? A cool $1.34 billion. Not too shabby for a company whose main product is under siege from copycats.
Speaking of copycats, let's talk about Eylea. The original formula saw revenues drop 21% YoY to $1.145 billion – that's what happens when biosimilars crash your party.
This is where it gets interesting though: Eylea HD (think of it as Eylea's muscled-up big brother) jumped from a mere $43 million to $392 million YoY.
Sure, about $40 million of that came from wholesalers stocking up like it's Black Friday at Costco, but still – that's what I call a growth story.
I've been watching Regeneron since they were just a gleam in Wall Street's eye, and they've always had a knack for turning scientific breakthroughs into cold, hard cash.
Take Dupixent, their inflammation blockbuster co-developed with Sanofi (SNY). It just got FDA approval for COPD with an eosinophilic phenotype.
Why does this matter? Because we're talking about a $6 billion market opportunity here, folks.
About 36% of COPD patients have this particular flavor of the disease and trust me, there are more of them than you'd think still wheezing away on their old inhalers.
Want to know what else is cooking in their labs? They're working on antibodies that could make blood clots a thing of the past – think better than Eliquis, which pulls in $10 billion annually for Pfizer (PFE) and Bristol Myers Squibb (BMY). Their secret? Something called Factor XI, which could be a game-changer for the 1 in 5 patients at high risk for bleeding.
And because no self-respecting biotech can resist the siren call of the obesity market, they're also cooking up their own weight loss cocktail. Results won't drop until late 2025, but if they crack the code on keeping weight off AFTER stopping treatment, they'll have something Wegovy and Zepbound can't match.
The financials are rock solid, too: $2.012 billion in cash, $7.785 billion in marketable securities, and current assets of $19.334 billion versus current liabilities of just $3.661 billion.
They've generated $3.158 billion from operations in the first nine months of 2024 alone.
Yes, there's $1.984 billion in long-term debt, but with cash flow like that, it's about as worrying as a paper cut.
I've already started nibbling at Regeneron, and I'm looking to add more if it dips further. After all, this is a company that's proven it can grow revenues at upper single digits year over year while maintaining 25% free cash flow margins - the kind of numbers that make a value investor's heart skip a beat.
Sure, there are risks lurking around every corner – biosimilars nipping at Eylea's heels, Medicare negotiations that could squeeze margins, and clinical trials that might go sideways.
But with multiple growth catalysts and a pipeline that reads like a wish list for modern medicine, Regeneron's got more upside than my daughter's college tuition bills.
As my optometrist likes to say - in the land of the blind, the one-eyed man is king. But in the land of biotech, Regeneron's got a 20/20 vision for what's coming next.