In a corner office somewhere in Cambridge, Massachusetts, a team of scientists is attempting something that sounds like it belongs in a sci-fi novel: they're trying to reprogram the genetic instructions that tell our neurons how to behave.
If they succeed, they might help 97% of people with ALS keep their neurons from self-destructing.
Welcome to Trace Neuroscience, where $101 million in venture capital is betting on what amounts to a molecular spell-check for your nervous system.
You might be wondering, as I did, how one starts a company with the audacious goal of tackling one of medicine's most notorious puzzle boxes.
The answer, it turns out, involves three scientists, working in three different labs, who all stumbled upon the same cellular culprit – a protein called UNC13A - the sort of name that makes you wonder if scientists moonlight as license plate generators.
But to understand why UNC13A has everyone buzzing, we need to talk about ALS itself.
ALS, if you're not familiar with it, is the kind of disease that keeps neurologists up at night. Every year, it claims about 5,000 new victims in the US alone, and we still don't know what causes 90% of cases.
Here's a simple way to envision it – think of your nervous system as a complex metropolitan subway system.
ALS is like having someone systematically shut down every station, one by one, until the entire network grinds to a halt. Despite decades of research and millions in funding, we're still mostly watching helplessly as stations go dark.
Sure, the global market for ALS treatments reached $667.3 million in 2023, but that impressive-sounding number masks an uncomfortable truth: we're still barely keeping the lights on, let alone fixing the underlying problem.
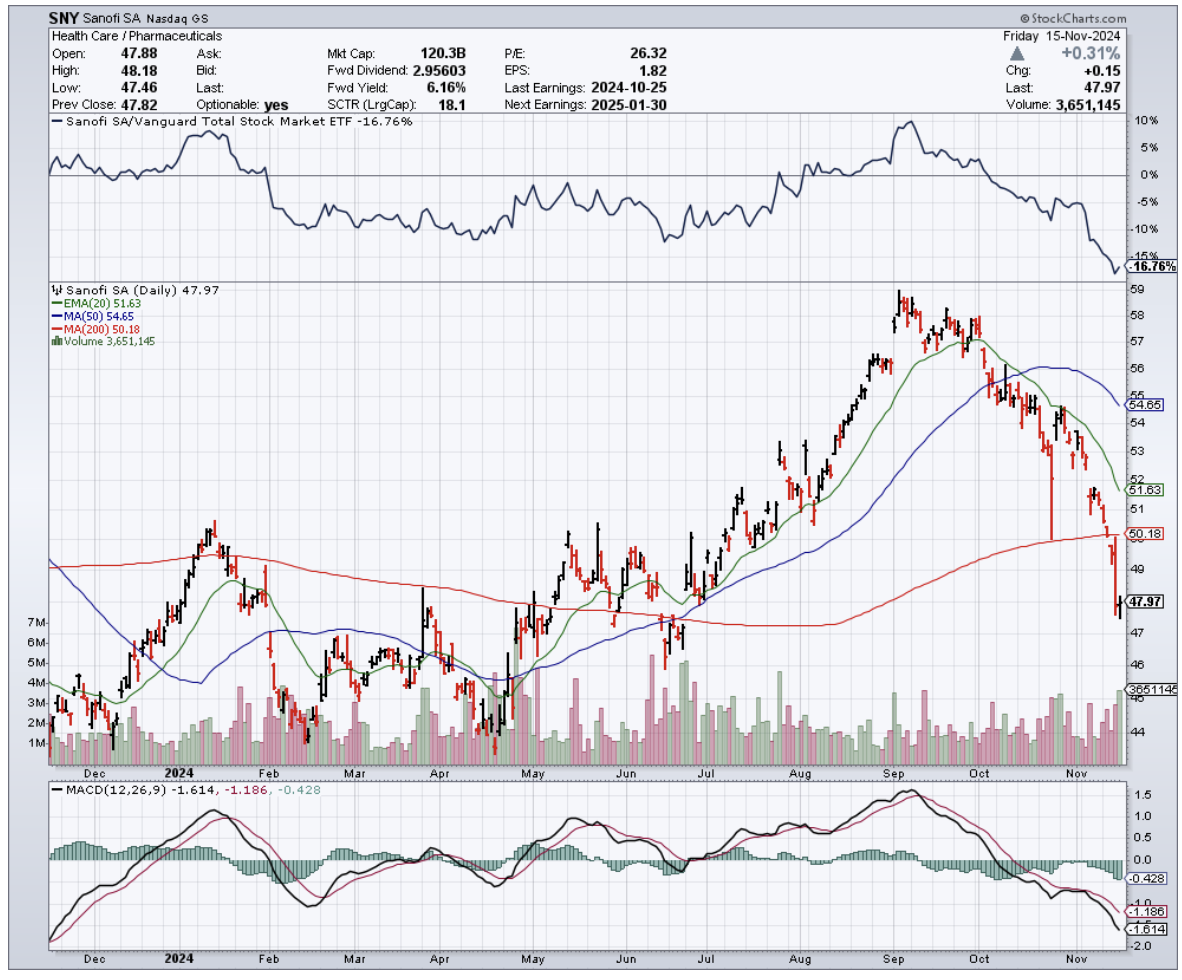
The current FDA-approved medications, Sanofi’s (SNY) Riluzole and Mitsubishi Tanabe Pharma’s (MTZPY) Edaravone (marketed as Radicava), are like trying to stop a flood with a handful of sandbags. They might slow things down a bit, but they're not exactly what you'd call a solution.
So how do you tackle a troublemaker like UNC13A? Enter Trace Neuroscience's bold approach: antisense oligonucleotides, or ASOs for those who don't enjoy tongue twisters.
Think of ASOs as tiny molecular scissors that can edit the body's protein-making instructions with surprising precision - in this case, they're specifically designed to fix how UNC13A behaves when it goes rogue.
The science behind this approach comes from a rather serendipitous confluence of research.
Aaron Gitler at Stanford, Pietro Fratta at University College London, and Michael Ward at the NIH – three scientists who probably should have just gotten a group chat going – independently discovered how certain RNA-processing molecules go haywire in ALS patients.
It's like they each found a different piece of the same puzzle, and when they put them together, the picture suddenly made sense.
And where there's breakthrough science in biotech, money usually follows.
In November 2024, Trace managed to convince some of the biggest names in venture capital – Third Rock Ventures, Atlas Venture, GV (formerly Google Ventures), and RA Capital Management – to part with $101 million.
That's quite a vote of confidence for a company whose main product is still theoretical.
The timing couldn't be more interesting. The ALS treatment market is expected to grow at a rather specific 5.8% per year until 2030, reaching about $1.1 billion.
But in this field, even the success stories come with asterisks.
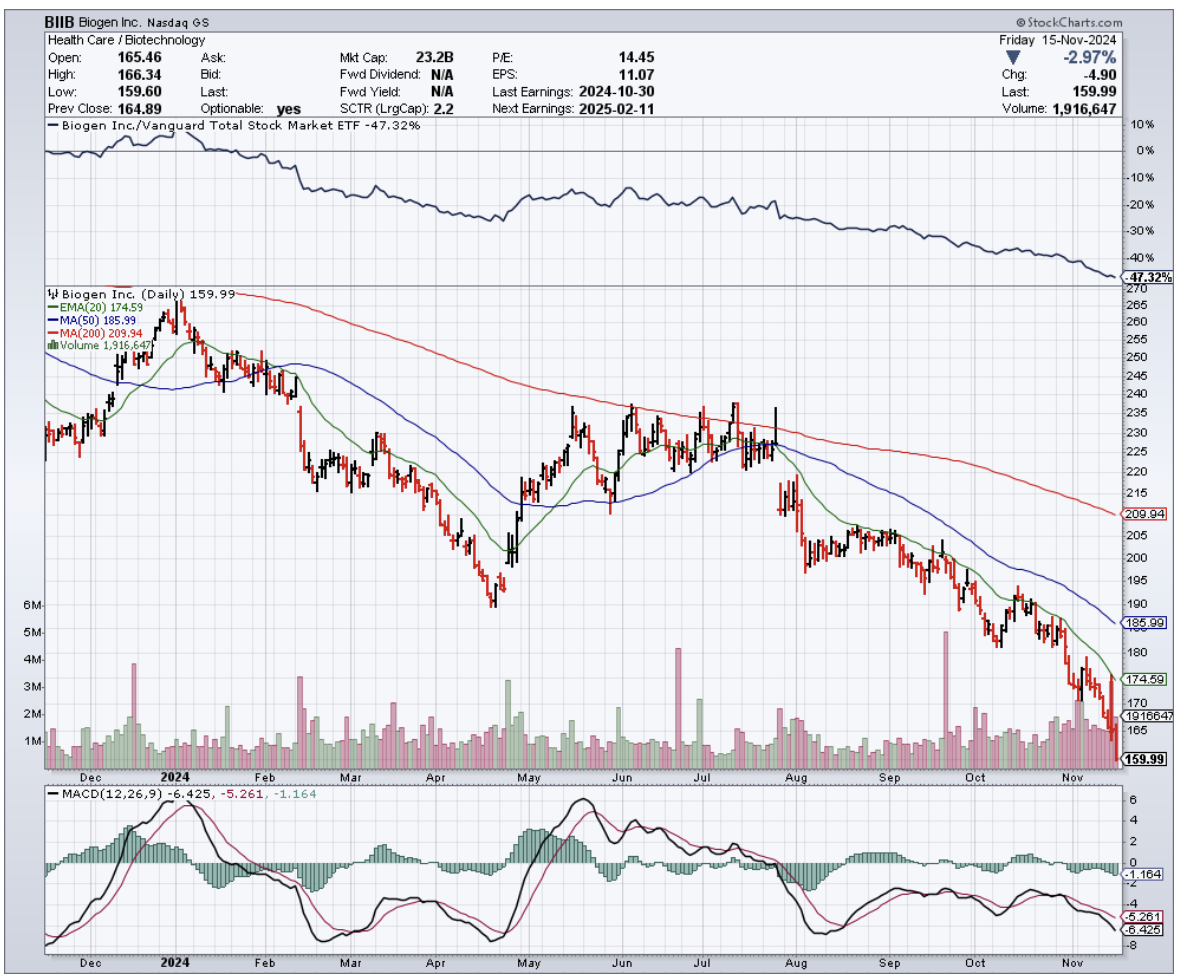
Take Biogen's (BIIB) Qalsody, which got FDA-approved in May 2024 despite not actually meeting its main trial goals.
It's a rare win in a field where the scoreboard has been mostly zeros, as the rest of the ALS treatment landscape makes painfully clear.
Ionis Pharmaceuticals (IONS) had to shut down their ALS program with Biogen in May 2024, and Amylyx Pharmaceuticals (AMLX) faced the bitter task of pulling their drug Relyvrio from the market in April 2024, laying off 70% of their staff in the process.
These setbacks illuminate an uncomfortable truth about ALS drug development: the path from lab bench to pharmacy shelf is paved with perfectly logical theories that simply didn't work in real bodies.
The math is brutal - only 6.2% of neurological drugs survive the journey from Phase I to approval. Trace's response to these odds? Assemble a team that's seen enough clinical trial failures to know how to (hopefully) avoid them.
Their CEO, Eric Green, M.D., Ph.D., leads a squad that includes Chief Medical Officer Irina Antonijevic and Chief Operating Officer Megan Baierlein.
They're aiming to start clinical trials by early 2026, which in drug development terms is practically tomorrow.
For investors, timing like this transforms Trace from a scientific curiosity into a near-term catalyst.
Their approach - anchored in genetic evidence and measurable biomarkers - stands out in a field where most companies are still shooting in the dark with better bullets.
The story of Trace Neuroscience reads like molecular medicine's version of going from medieval star-gazing to GPS navigation.
While traditional ALS treatments chase symptoms, Trace is tracking proteins with the precision of an atomic clock.
They've transformed one of medicine's most frustrating puzzles into something remarkably concrete: either their molecular markers will move, or they won't.
In the biotech world, that kind of clarity is worth watching.