When Silence Is Golden
I was halfway through my morning coffee when my trader buddy in New York called me at some ungodly pre-market hour. He's one of those Wall Street guys who never sleeps and hasn't taken a real vacation since the Reagan administration.
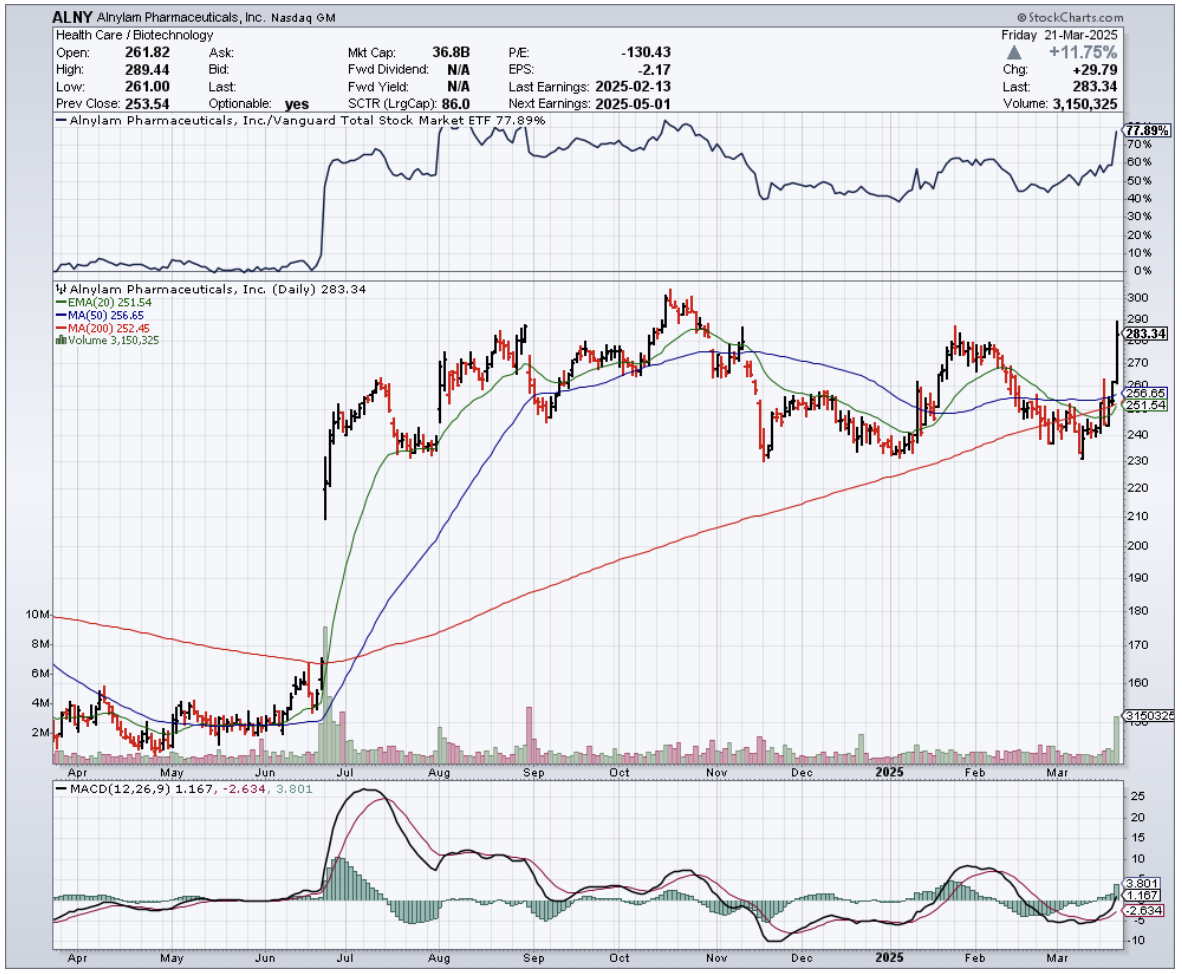
"So what's your take on Alnylam?" he barked, not bothering with pleasantries. "Stock's been trading sideways for months between $230-$300. I'm getting impatient."
I took another sip of my Kona blend, remembering why I left the trading floor years ago. "Let me guess – you're looking at your Bloomberg terminal right now instead of enjoying the sunrise?"
"Cut the Zen master crap, John. What's the play here?"
The reality is that Alnylam Pharmaceuticals (ALNY) just snagged FDA approval for Amvuttra in ATTR-CM, opening the door to potential revenue north of $6 billion. But my friend, like most traders, was looking for the angle that wasn't already priced in.
"You know," I said, "when I was a combat pilot, we had a saying: 'It's not the missile everyone sees coming that gets you.' The approval announced on March 20th was expected, sure. But the label is better than most anticipated – approved for both hereditary and wild-type ATTR-CM. It's the first drug green-lighted for both polyneuropathy and cardiomyopathy, with the latter being more common and potentially more lucrative."
The label includes language about reducing "cardiovascular mortality, cardiovascular hospitalizations, and urgent heart failure visits" – with that last bit about urgent heart failure visits exclusive to Amvuttra. Seems small, but in pharmaceutical marketing, these distinctions matter.
"So should I buy the stock or not?" he pressed, always impatient.
"Here's what the Wall Street research notes won't tell you," I continued. "The HELIOS-B clinical study was a home run. Amvuttra will eventually muscle its way into front-line treatment, but initially, expect it to grab the 30% of ATTR-CM patients who deteriorate on Pfizer's (PFE) tafamidis or BridgeBio's (BBIO) acoramidis. Those are the 'stabilizers,' while Amvuttra is a 'silencer' – and in this case, silence is golden."
I could hear him typing furiously. "What about patient switchovers from stabilizers?" he asked.
"Doctors hate changing treatments that work, even if something better comes along. It's like trying to convince an old trader to use an app instead of calling his broker – not happening unless there's overwhelming evidence. But there's another angle – Amvuttra requires only four injections yearly versus daily pills. Plus, it's covered under Medicare Part B with zero copays, while the others fall under Part D. When patients realize they can save thousands in out-of-pocket costs, watch what happens."
Management is sticking with their hefty list price of $476,000 per year, though the real-world price after rebates and discounts will be lower. Given the clinical data and market dynamics, I'm convinced this indication alone is worth $6+ billion to Alnylam, with an upside to $7-8 billion if their newest compound, nucresiran, maintains efficacy with twice-yearly dosing.
"Fine, but is this a one-hit wonder or do they have more in the pipeline?" he pressed.
"Their R&D engine is firing on all cylinders. They've expanded beyond the liver to target neurodegenerative and ocular diseases, with plans to cover all major tissue types by 2030. Their batting average is impressive – three self-commercialized drugs, one partnered drug on the market, and another likely approval coming soon via Sanofi (SNY). That's the kind of success rate that makes venture capitalists weak in the knees."
I won't sugarcoat it though – currently, about 75% of their revenue comes from the TTR franchise, with Givlaari and Oxlumo making up the rest. Diversification is coming, but it's not an overnight process.
Keep your eye on zilebesiran, their antihypertensive being developed with Roche (RHHBY). Early results look promising, with KARDIA-3 results due later this year. It won't move the revenue needle immediately, but could eventually contribute significantly.
There's also mivelsiran for cerebral amyloid angiopathy and Alzheimer's – still in Phase II but potentially worth billions if successful. Not to mention early-stage programs targeting Huntington's, bleeding disorders, diabetes, obesity, and AMD. The pipeline is loaded like a billionaire's stock portfolio.
"Bottom line it for me, John. I've got a morning call in five minutes."
"Management's guiding for 30% product sales growth in 2025, mostly from Amvuttra in ATTR-CM. Their forecasts tend to be conservative but achievable – not the pie-in-the-sky numbers you get from pre-revenue biotechs promising to cure cancer with fairy dust."
In my model, the TTR franchise contributes about $202 per share to my $312 fair value estimate. Zilebesiran adds roughly $35, with an upside beyond $50 if clinical data continues to impress.
Admittedly, a 10% upside isn't the sexiest call for a biotech stock. But after watching thousands of companies come and go during my decades on Wall Street, I've learned to appreciate the rare combination of proven technology, commercial products, and pipeline depth that Alnylam offers.
"So you're saying buy?" he asked, clearly rushing now.
"I'm saying Alnylam isn't the stock you brag about at cocktail parties, but it might just be the one that pays for your kid's college education. Speaking of which, when was the last time you saw your children?"
He hung up without answering. Some things never change on Wall Street.