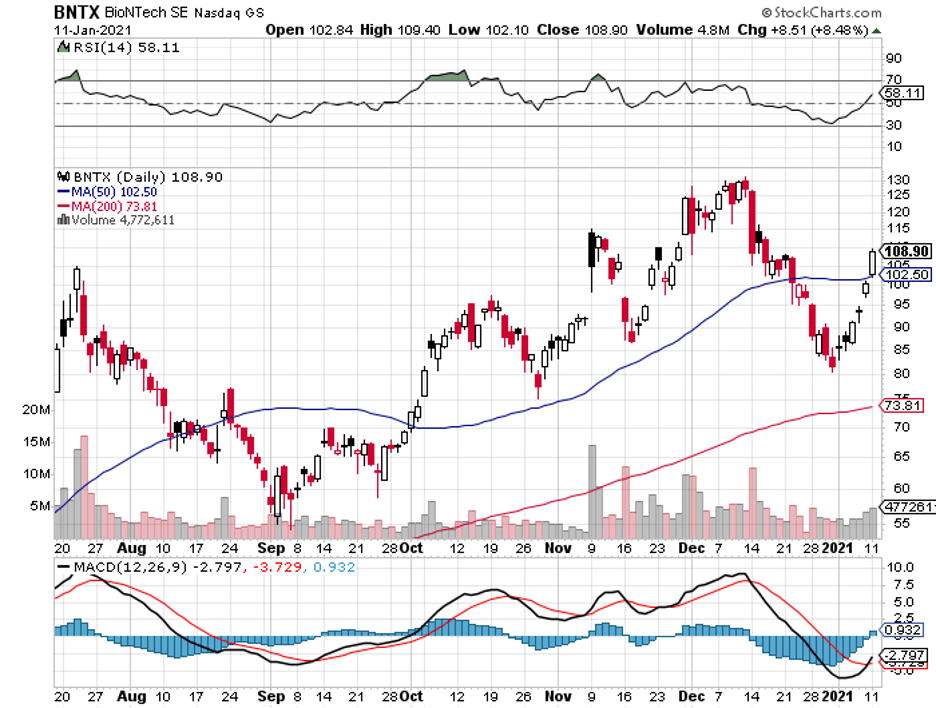
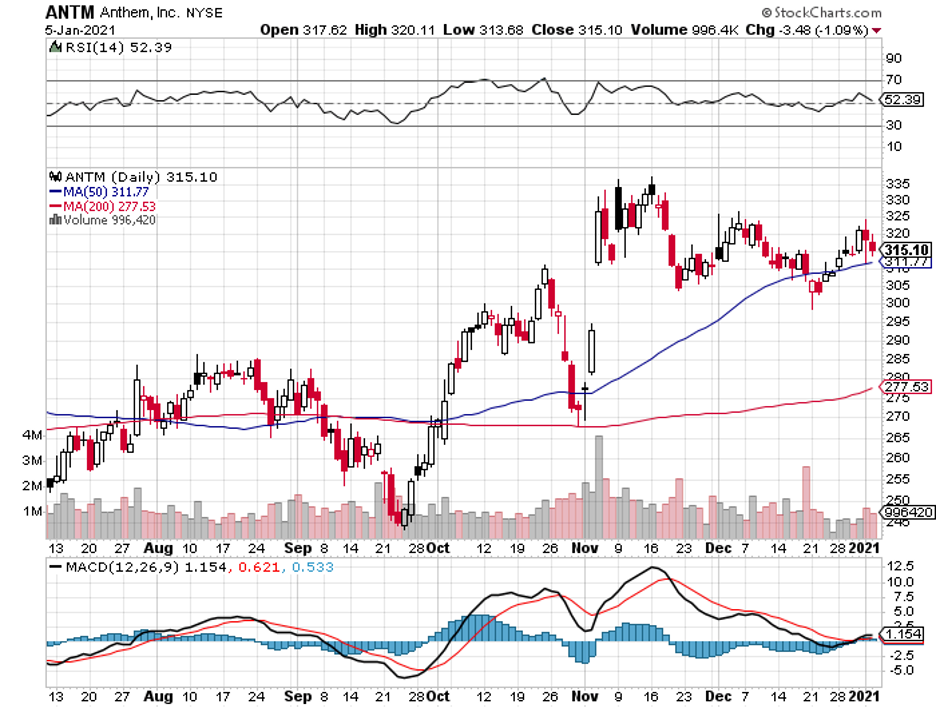
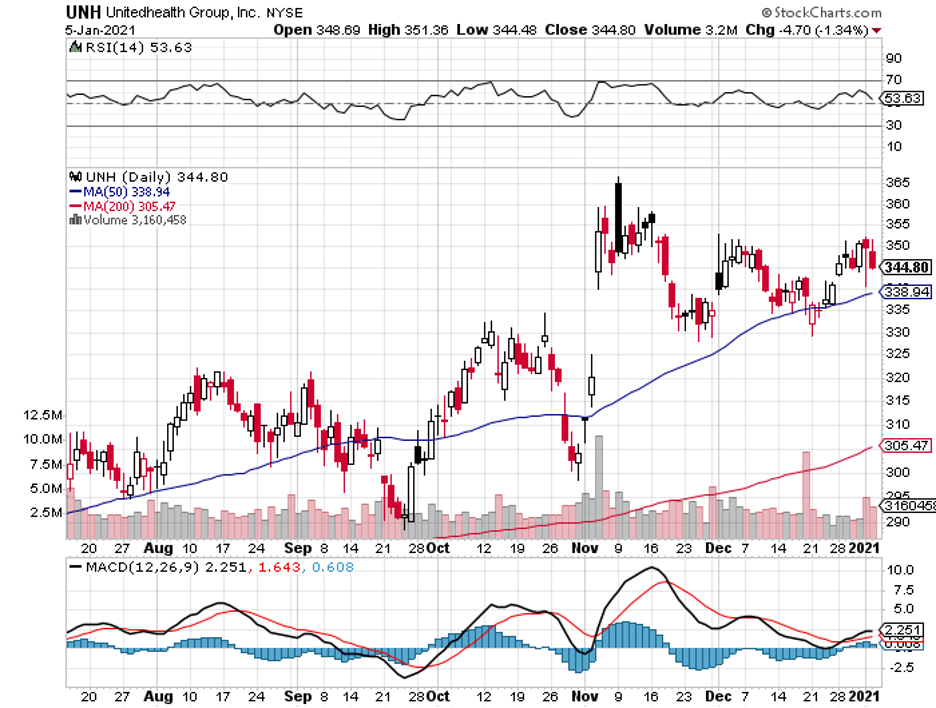
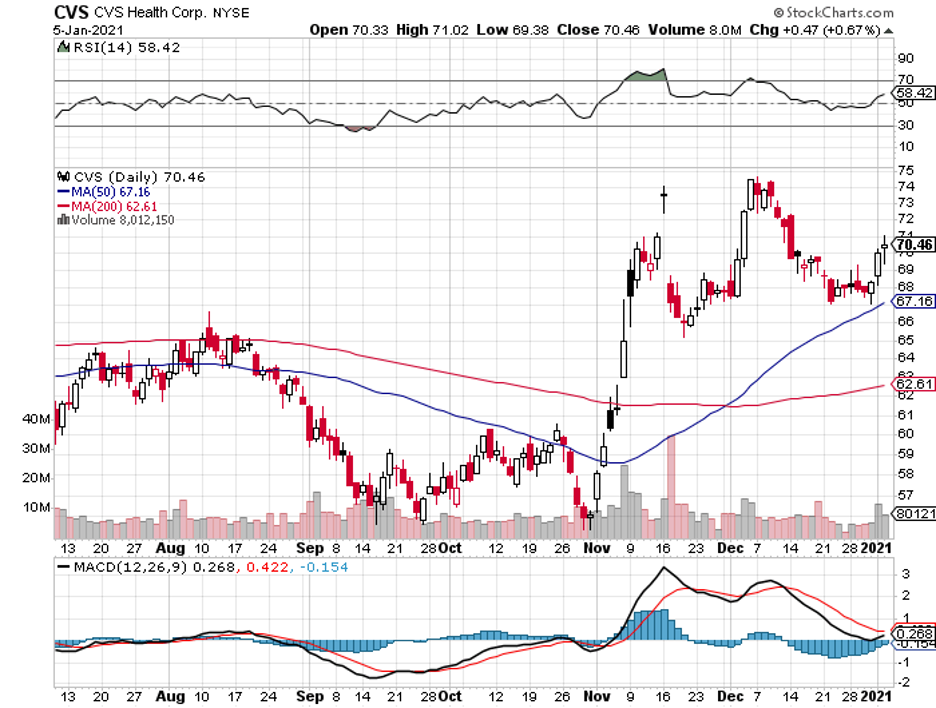
To date, some coronavirus vaccine stocks have managed to offer investors huge gains within short periods.
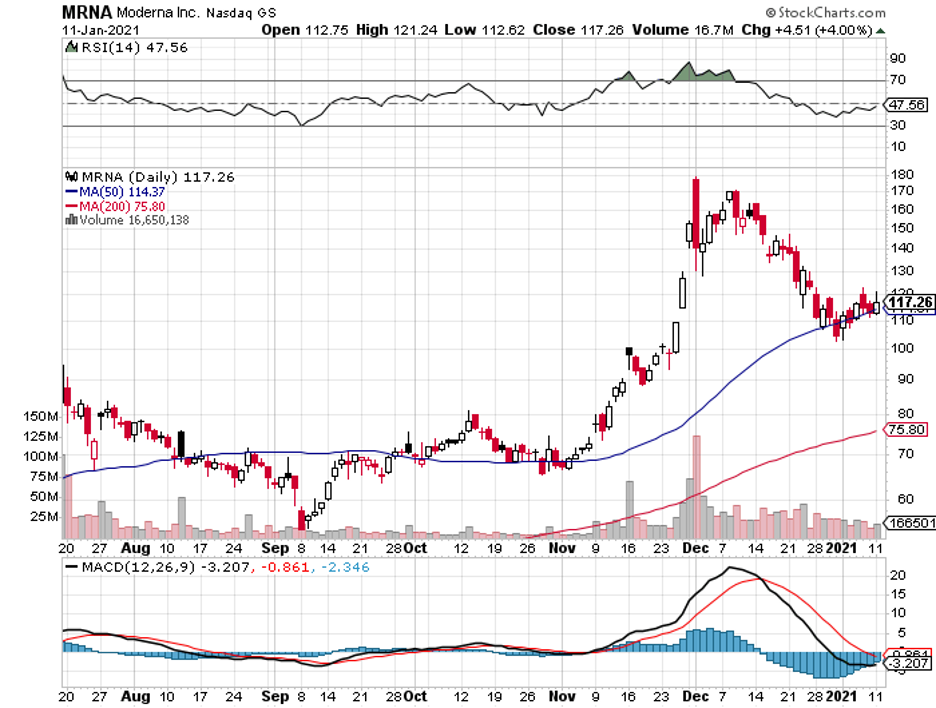
Moderna (MRNA) is a prime example, climbing to over 400% in 2020 as more and more investors bet on the biotech’s COVID-19 program.
Interestingly, Pfizer (PFE) fell by less than 1% during the same period despite the fact that both companies led the COVID-19 vaccine race.
Actually, it was Pfizer that eventually won the first Emergency Use Authorization.
Investing in COVID-19 vaccines brought about vastly different outcomes.
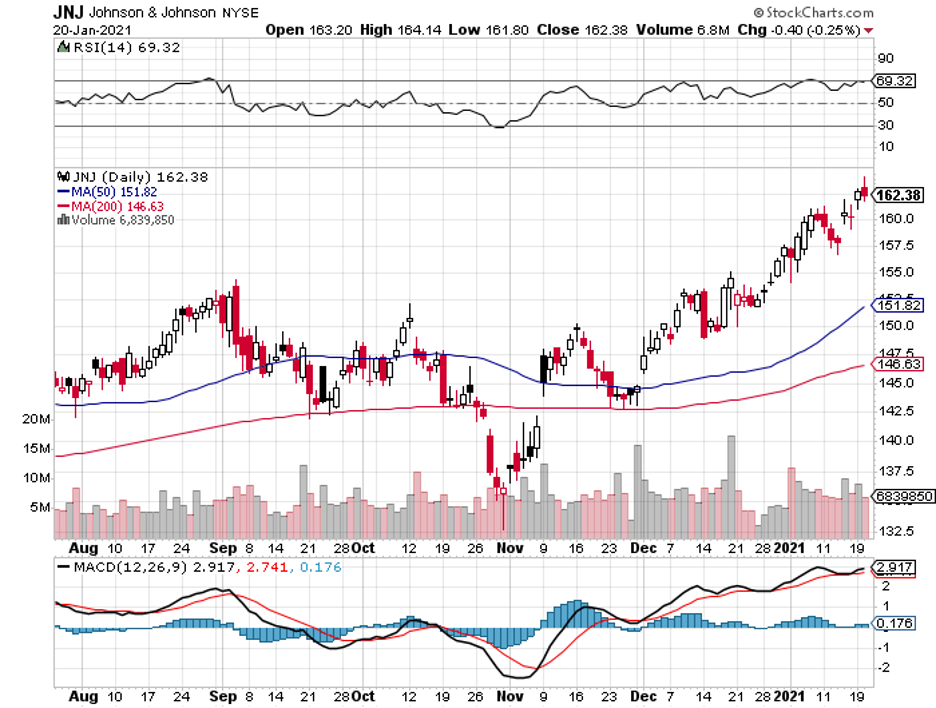
Now, let’s take a look at the next company to potentially enter the market: Johnson & Johnson (JNJ).
At the moment, JNJ’s candidate is in Phase 3 trials. What makes this trial interesting is the dosing regimen.
JNJ is evaluating two options in its Phase 3 trials.
The first option, called “Ensemble,” studies the effect of a single dose of its COVID-19 vaccine.
The second option, “Ensemble 2,” examines how the vaccine works when administered in two doses.
Results are expected to be released before January 2021 ends.
If all turns out positive, then JNJ will be the third company to cross the COVID-19 vaccine race finish line.
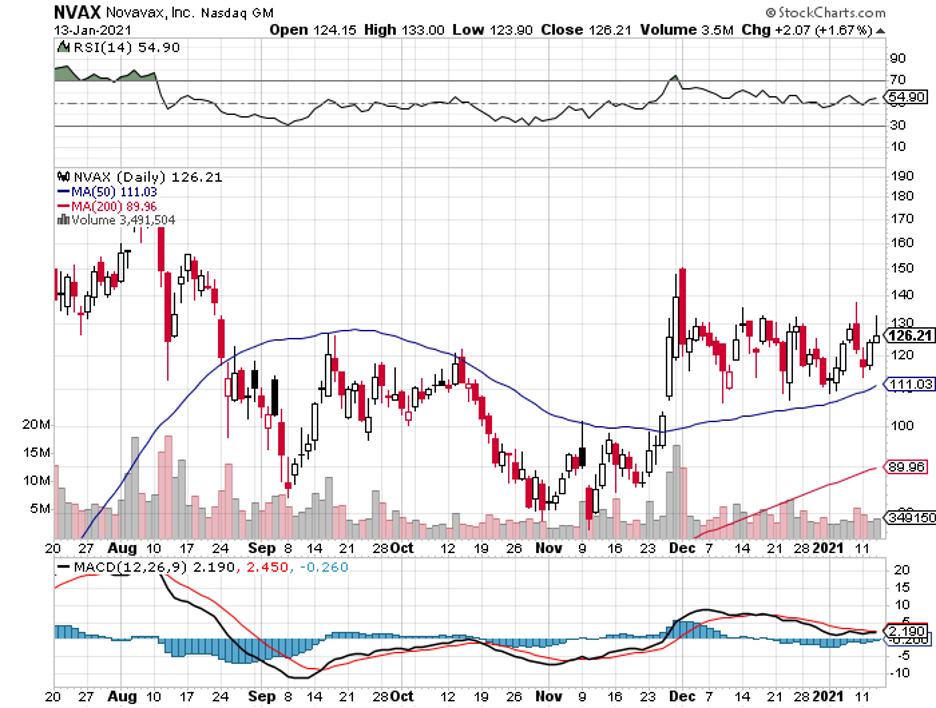
Both Moderna and Pfizer require two doses. Even its close-to-market competitors like AstraZeneca (AZN) and Novavax (NVAX) have developed two-dose products.
Needless to say, a proven safe and effective one-dose regimen would provide JNJ’s vaccine a significant competitive advantage.
Obviously, people would prefer the idea of a one-jab vaccine instead of getting two. More importantly, this regimen would make it easier to vaccinate more individuals.
For example, Moderna’s goal is to supply 1 billion doses in 2021. However, that will only cover half or 500 million people.
In comparison, JNJ’s promise of producing 1 billion doses per year will be sufficient to immunize 1 billion individuals.
Another advantage is JNJ vaccine’s storage requirement.
Unlike Pfizer and Moderna’s vaccines, JNJ’s candidate can be stored at refrigerator temperatures for at least 3 months.
Taking all these into consideration, JNJ’s vaccine is looking promising.
Now, let’s check out its revenue potential.
Taking cue from AstraZeneca, JNJ has announced its decision to offer its vaccine on a not-for-profit basis during the COVID-19 pandemic.
This means it plans to sell the product at cost.
Without any profit from this venture, we can’t count on the COVID-19 vaccine to add to the company’s earnings in the near future.
Post-pandemic, though, the company could eventually raise the price and start benefiting from its sales.
While there’s no definitive timeline, this could be around the later parts of 2021. Based on that prediction, JNJ could start benefiting from its COVID-19 vaccine sales by early 2022.
JNJ can easily become a leader in the coronavirus market if its one-dose vaccine gains regulatory approval from health agencies across the globe.
However, I don’t expect major gains in its share price even if it does happen.
From what I’ve observed so far with Moderna and Pfizer, the shares of clinical-stage biotechnology companies tend to soar while those of bigger biopharmaceutical counterparts do not.
That’s most likely because the smaller companies will rely heavily on the COVID-19 vaccine revenue while the more established companies, with their extensive range of commercialized products, won’t.
With the sheer size of JNJ, which has a market capitalization of over $427 billion, its shares seldom make huge moves.
Still, JNJ continues to show incremental and steady growth in profits, revenue, and even share price.
In fact, the company is up by over 10% over the previous year. More importantly, investors can always count on the company for dividends.
So, I wouldn’t buy JNJ shares only for its COVID-19 vaccine candidate. It’s not the best idea to buy a stock because of a single product anyway.
But, I would definitely buy JNJ shares for its overall portfolio and its ever-reliable increasing dividend payouts.