Over 5.8 million people in the United States live with Alzheimer’s disease, and there are at least 487,000 new cases recorded every year.
Sadly, there has been no new treatment approved for this condition since 2003.
It isn’t for the lack of trying though.
In fact, large-cap biotechnology companies like Pfizer (PFE), H Lundbeck A/S (HLUYY), and Eli Lilly (LLY) have tried their hands at coming up with a drug to treat Alzheimer’s disease.
Unfortunately, none of them succeeded.
Amid the failure of these industry giants to develop a cure, a small-cap biotechnology company based in Austin, Texas has emerged with a potential answer to the problem.
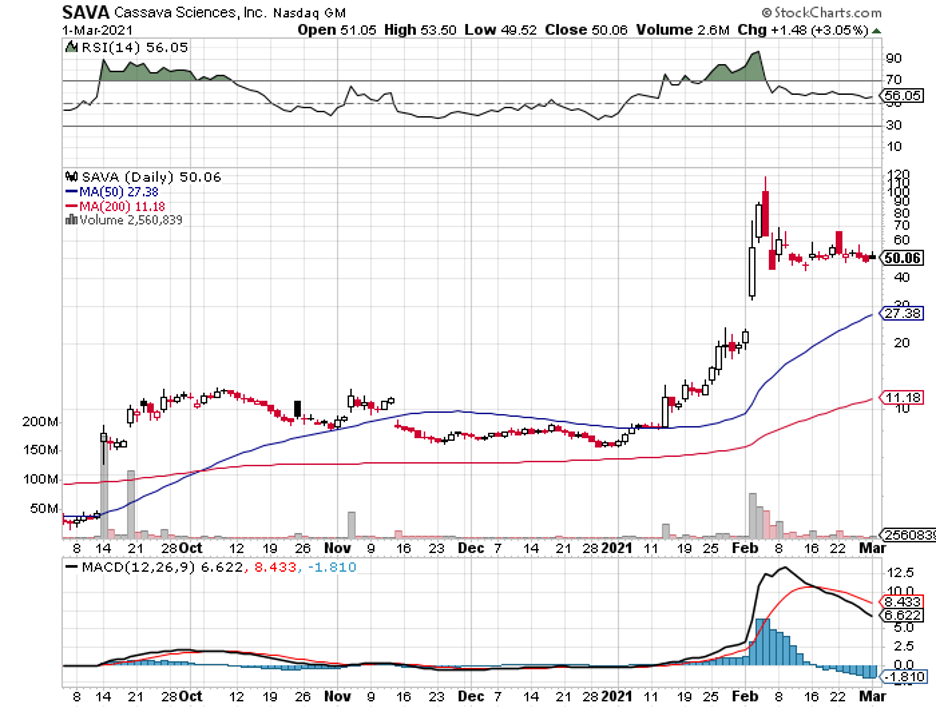
Cassava Sciences (SAVA), which has a market capitalization of $2 billion, is offering investors a different direction—and its efforts haven’t gone unnoticed.
Over the past 12 months, Cassava stock rose by a whopping 668%.
The overwhelming interest in the stock is understandable.
In February, Cassava released promising reports about its own Alzheimer’s drug candidate, Simufilam.
Patients who took Simufilam for six months showed 10% improvement on their cognition tests, while their dementia-related behavior improved by 29%.
The next stage would be for Cassava to go through Phase 3 of the study for Simufilam.
Interestingly, the success of Simufilam’s trials has not only benefited Cassava but also several smaller biotechnology companies working on Alzheimer’s disease treatments.
Specifically, Anavex Life Sciences (AVXL), which only has a market capitalization of almost $900 million, gained an impressive 129.4% boost.
Meanwhile, Cortexyme (CRTX), which has a market capitalization of $1.07 billion, rose by 57.8% this year following the positive data release.
While Cassava’s results are definitely worth looking into, it’s critical to understand the limits of the data the company has provided the public thus far.
My caution against Cassava at this point is not based on the belief that its Alzheimer’s disease program will fail.
Rather, I’m wary of the stock because its value right now is heavily based on the misunderstood perception that Simufilam has already succeeded.
Looking at the current data from the company, I believe that the skyrocketing price at this point remains unjustified.
It’s important to keep in mind that the FDA will not grant approval to a drug unless it shows satisfactory effectiveness in Phase 3 clinical trial.
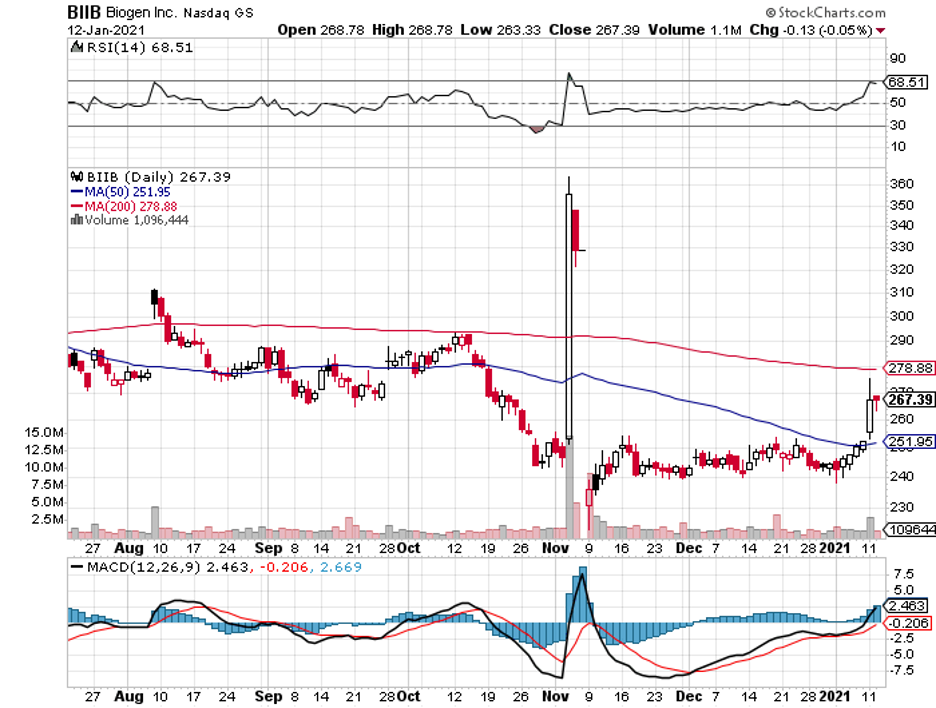
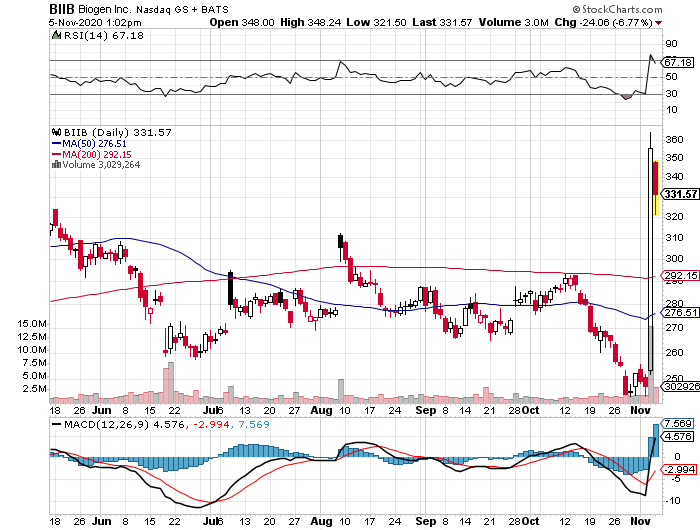
A fairly recent example of a cautionary tale is the fanfare generated by Biogen (BIIB) when it released promising data for its own Alzheimer’s drug, Aducanumab.
However, this isn’t to say that Simufilam won’t make it, or that it will experience the same issues faced by Biogen.
This simply means that valuing this stock requires a more sober assessment. It’s challenging to determine its actual value right now with all the speculative fever surrounding it.
Remember, clinical trials for Alzheimer’s disease would set a company back roughly $1.8 billion on average.
It also typically takes more than four years to complete. At this point, Cassava only has approximately $94.3 million in cash.
This means it would need to either land a development partner to help shoulder the expenses or sell additional stock to come up with additional funds.
The Alzheimer’s drug market is massive, which is a clear indicator of the dire need in this space because there remain no reliable drugs available.
On the low end of the estimate, the global Alzheimer’s drug sales is projected to be $3.5 billion back in 2018.
On the high end, the number could reach $4.9 billion in 2013 to over $13.3 billion by 2023.
What are the prospects of an effective Alzheimer’s disease drug? Let’s go back to Biogen.
Its Aducanumab, which never managed to release impressive data, still estimated peak sales of roughly $4.2 billion.
Back of the envelope math says that an approved, safe, and effective treatment would undoubtedly generate blockbuster multi-billion dollar sales.
After all, large-cap companies pay a premium for exclusive rights to promising drugs.
To use an approved exclusive drug as an example, let’s take a look at the September 2020 deal between Immunomedics and Gilead Sciences (GILD).
Prior to the deal, Immunomedics developed an exclusive and promising chemotherapy drug called Trodelvy.
Like Aducanumab, that treatment was valued to rake in $4 to $5 billion in peak sales.
Seeing the potential, Gilead Sciences bought out Immunomedics to get Trodelvy.
The deal? It was worth $21 billion, or approximately 100x where Cassava trades when 2021 started.
Although it’s difficult to determine how much Cassava would eventually be valued, the sales for its Alzheimer’s drug should project better numbers than the regularly doubted Aducanumab.
The bottomline is this: Cassava is a promising stock that offers an Alzheimer’s disease drug candidate that reported better results than what the big players in the industry achieved so far.
Investors should expect volatility from this company in the next few months or even years as it enters a crucial stage: the Phase 3 trials, otherwise known as the drug development graveyard.