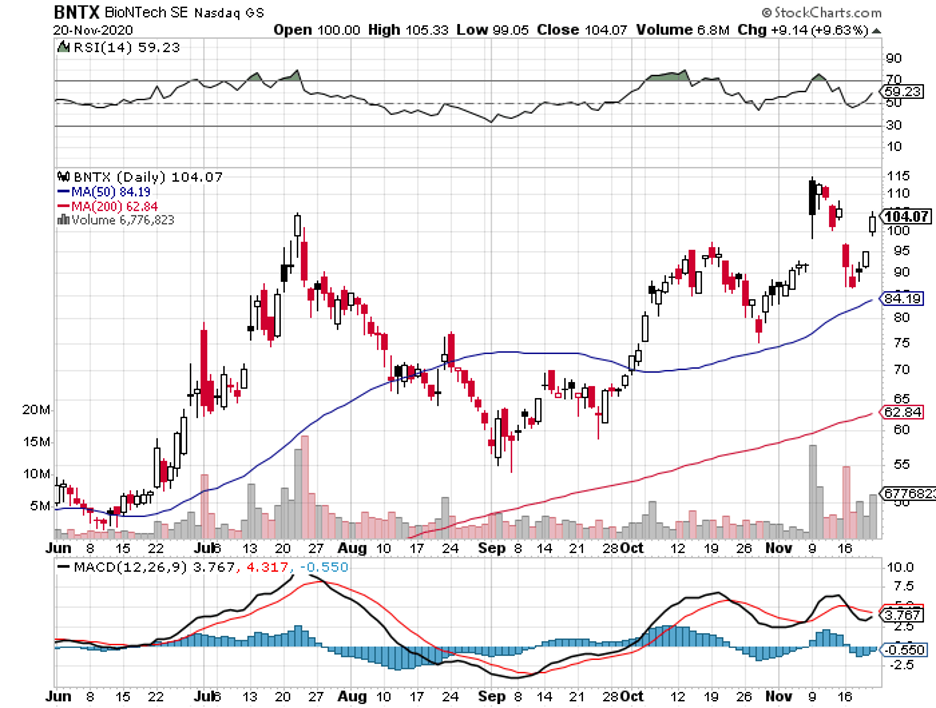
BioNTech (BNTX) is the perfect example of an old saying, “Timing is everything.”
Coming from its humble IPO in 2019, this biotechnology company now sports a $25 billion market capitalization—a number that could still go up once its COVID-19 vaccine candidate with Pfizer (PFE) receives US and EU nods.
What we know so far is that their COVID-19 vaccine candidate could secure an emergency approval as early as December and start delivery before Christmas.
Although it’s still not available in the market, the effect of its COVID-19 vaccine candidate, called BNT162, has made itself known in BioNTech’s earnings report.
The company reported roughly $80 million in revenue in the third quarter of 2020 alone—an impressive 135% jump from its previous performance in the same period last year.
To date, BioNTech and Pfizer are estimated to supply roughly 1.3 billion doses by the end of 2021.
Additional orders could still come in though, which is why the two companies have been busy scaling their manufacturing capacities.
If all goes according to plan, then the expected returns from their COVID-19 vaccine sales could come sooner than initially thought.
Recent reports reveal that Moderna’s (MRNA) COVID-19 vaccine candidate also showed over 90% efficacy. Even AstraZeneca’s (AZN) candidate with Oxford University disclosed promising results.
However, BioNTech and Pfizer’s candidate has a couple of competitive advantages.
The first would be its 95% efficacy, which gives the two companies the commanding position and effectively relegates the rest as second grade options.
Their candidate showed no safety concerns—a major issue for AstraZeneca and Johnson & Johnson’s (JNJ) candidates.
Third, the partners have been able to reassure their capability to manufacture at scale—an issue that would pose problems for other developers like Moderna.
In fact, BioNTech acquired a vaccine manufacturing plan in Germany just last September to meet the demand for 250 million doses by mid-2021 and another 80 million doses monthly thereafter.
In terms of manufacturing capacities, the two potential competitors of BioNTech and Pfizer here are AstraZeneca and JNJ. Both have already paused their trials and are now falling behind in terms of the rigid schedule.
As for the other COVID-19 vaccine leader, Moderna has yet to prove that it can manufacture at scale.
BioNTech and Pfizer even shut down the red herring about the cooling and storage of their COVID-19 vaccine candidate. The two companies released their plans for distribution and detailed a strategy that’s not only feasible but also cheap.
Since the vaccine requires extremely low temperatures to maintain its efficacy, Pfizer and BioNTech will ship them from centralized warehouses via a thermal shipper.
This will ensure that the temperature is maintained for 10 days without the need to re-ice and up to 15 days with re-icing. A GPS will be used to monitor and track the integrity of the vaccine in real-time.
The impact of its sales from the COVID-19 vaccine would dwarf practically everything else in BioNTech’s financial statements.
However, this does not mean the biotechnology company will revert to its 2019 status once the peak of its COVID-19 vaccinations is over.
Instead, BioNTech will be in possession of an extremely valuable IP of an effective and working mRNA vaccine platform.
This will allow the company to apply the technology to other infectious diseases.
If it continues with its partnership with Pfizer, it can even develop vaccines for farm animals and domestic pets and market those under the bigger company’s animal healthcare spinoff, Zoetis (ZTS).
Here’s a bit of background on BioNTech.
Founded in 2008, BioNTech was created to develop hyper-personalized medicine and treatments.
At the center of its mission, the company’s basic idea is that the tumor found in each cancer patient is one of a kind.
To help find a cure or treatment, the company analyzes the tumor for its genetic signature.
Once they identify this unique element, they would develop gene-based therapies to limit the spread or even put an end to that particular occurrence of cancer.
If you think this is a lofty goal for a small biotechnology company, then you’d be surprised to find out that BioNTech proved their theories in 2017.
At the time, all 13 patients who underwent the analysis and received injections for genetically personalized therapies for their advanced-stage cancers.
Essentially, the cancer patients developed immunity from their own cancer.
Apart from COVID-19 and cancer treatments, BioNTech is also working on treatments for tuberculosis, HIV, and several rare diseases.
Outside its partnership with Pfizer, it has been partnering with Regeneron (REGN) and Genentech (DNA).
Biotechnology stocks have the tendency to move when the companies release updates about their treatments under development.
For momentum investors, it’s crucial to be prepared for whatever happens in the aftermath.
Looking at the developments and other updates, BioNTech’s COVID-19 vaccine work could send this stock to the moon.
After all, its partnership with Pfizer resulted in what could be the most effective and efficient candidate to battle the pandemic.
This means that the demand for the vaccine would exponentially exceed the supply in the near future, with the majority of what can be manufactured getting pre-sold or call option reserved.
To date, BioNTech stock is trading at roughly $104 per share. However, I estimate that it could reach a target price of $600 by the first quarter of 2021.