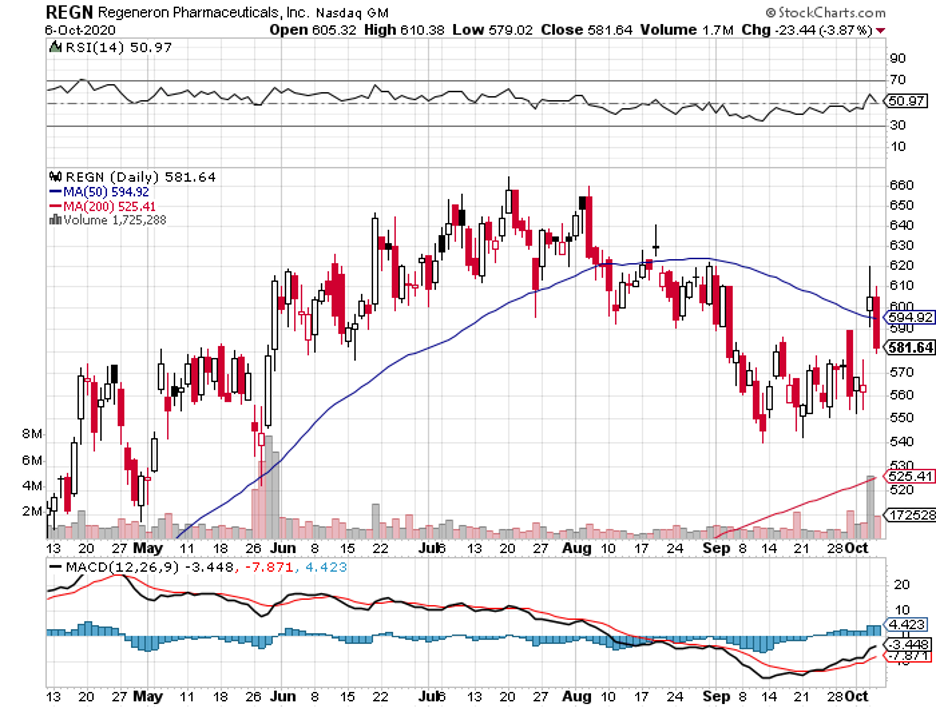
If the experimental COVID-19 treatment of Regeneron Pharmaceuticals (REGN) is good enough for the US president, then this stock should be given more attention not only by the media but also by investors.
One of the biggest stories this October is that President Donald Trump got infected with COVID.
The bigger story for the stock market though is his choice of treatment.
According to his medical team, Trump was given Regeneron’s antibody cocktail, called REGN-COV2, which was actually developed based on the same technology used in the company’s experimental Ebola treatment.
Although REGN-COV2 is still in the trial phase, reports that Trump already beat COVID just three days since his diagnosis are doing wonders for the stock.
Apart from REGN-COV2, Trump also received Gilead Sciences’ (GILD) Remdisivir as well as dexamethasone, a common generic steroid he once touted as a “miracle COVID-19 cure.”
The president was given aspirin and famotidine, which is more widely known as Johnson & Johnson (JNJ) and Merck’s (MRK) Pepcid.
On top of these, he took zinc, Vitamin D, and two immune-boosting supplements.
Compared to how far Gilead’s Remdesivir has gone in terms of offering treatment to COVID-19 patients with severe symptoms, Regeneron’s candidate is nowhere near the finish line.
Among all these drugs, however, Regeneron enjoyed the most advantage, with its stocks rising to roughly 5% since the announcement. Gilead also experienced a boost from the news, with a 3% jump.
What does this mean for investors?
Well, this news triggered aggressive buying of Regeneron shares. As expected, the unusually heavy volume pushed the stock price up.
While it would be tempting to join the market mob in buying a hot stock in the hopes of it getting even hotter, you might want to consider switching gears instead.
Hot stocks that dominate the news tend to cool and end up sliding at some point.
Rather than buying Regeneron stock right now, think about buying its bullish call options.
Options are always cheaper than their associated stock, which means you’ll be less at risk if something happens that lowers the stock price.
Even if the stock continues to advance, investing in options will still ensure that you get a nice return.
After all, each options contract represents 100 shares of stock.
To date, Regeneron’s stock is up 7.2% at $605.
That means you should buy bullish November $600 call options for roughly $40 with the expectation that REGN-COV2 gets approved—or at least stays as a strong contender until the next earnings report.
Since Regeneron released its 2019 third-quarter earnings report on November 5, it’s reasonable to assume that the company will follow the same timeline for 2020.
Therefore, setting the expiration to November ensures that you cover its third-quarter earnings report this year.
Aside from that, you’ll have enough time to gauge the success of REGN-COV2 and how the results will affect the stock price.
If the company’s share price reaches $665 at the expiration date, which is its peak price in the past 52 weeks, the call would be worth $65. If it hits $700, then the call will be worth $100.
For context, Regeneron stock has been anywhere between $279.22 and $664.64 in the past 52 weeks.
If REGN-COV2 gains approval, its projected 2021 sales could reach $1.8 billion. Meanwhile, its 2022 sales could hit $2.4 billion, with a decline to $1.7 billion by 2023.
Outside its COVID-19 efforts, the company has a promising portfolio to keep investors interested.
Regeneron’s annual revenue for its marketed drugs has been consistently climbing since 2012, with the biotechnology company’s earnings beating estimates in the last four quarters.
At the moment, the company has over 30 programs in its pipeline, 9 of which are in Phase 3, ensuring that its portfolio still has so much room for growth.
At the height of the pandemic, Regeneron maintained its stellar balance sheet in the second quarter.
One of its top-selling drugs is atopic dermatitis medication Dupixent, which it developed with Sanofi (SNY), with $770 million in sales for that period alone.
Looking at the drug’s track record, Dupixent is projected to rake in $6.3 billion in sales in 2021.
However, the top performer in the second quarter is eye injection Eylea, which contributed $1.1 billion in sales.
Meanwhile, skin cancer treatment Libtayo generated $63 million and cardiovascular disease drug Praluent raked in $47 million.
Regeneron also finished the second quarter with $943 million in net cash flow, which is a massive jump from the $188 million it reported in the same period in 2019.
On top of Regeneron raking in huge rewards for ’s COVID-19 treatment if approved, the company also has other promising products in its portfolio—ones that can still sway investors in their favor regardless of REGN-COV2’s future.