As we hold our breath for the results of the presidential election, it’s no surprise that investors are wondering how their portfolios will be impacted.
That’s why now is the right time to pick a stock or two that can thrive regardless of who emerges as the victor.
To do this, it’s wise to look at a company that has already experienced a boost under Trump’s presidency and could continue to enjoy the rewards even with a Joe Biden administration.
The obvious common denominator is Trump and Biden’s goal to be aggressive in COVID-19 testing for as long as the virus is around.
Pfizer (PFE), AstraZeneca (AZN), and Moderna (MRNA) are undoubtedly three of the most widely reported coronavirus stocks in the past months.
These companies were the first to launch their COVID-19 vaccine candidates in human trials and are the leaders in the race towards the finish line.
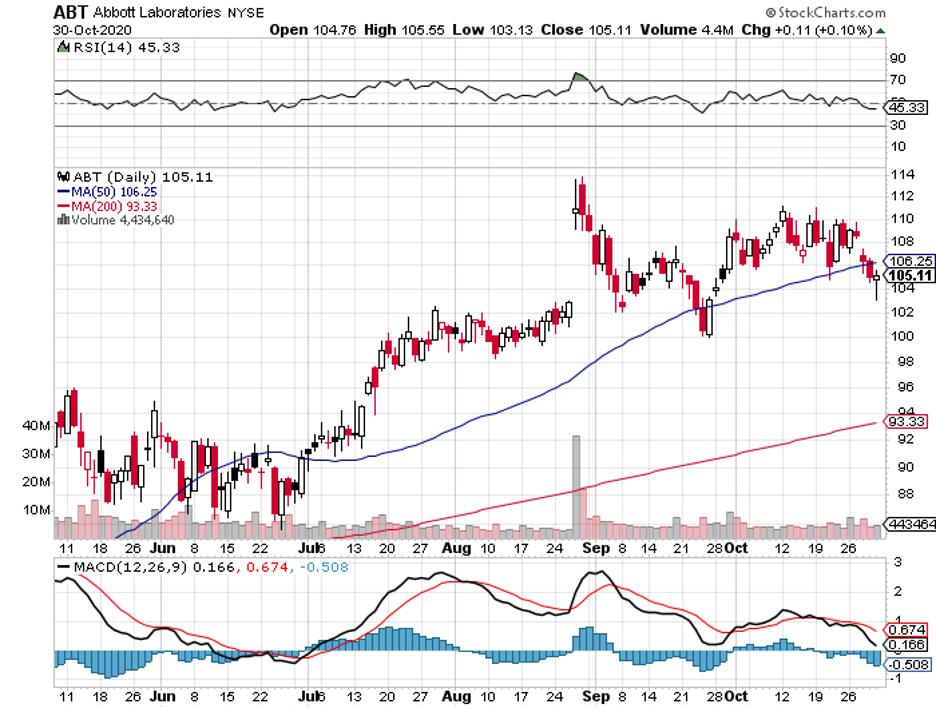
However, long-term investors may find more value betting on one of my preferred COVID-19 stocks: the $188 billion healthcare behemoth Abbott Laboratories (ABT).
Let me tell you why.
In either Trump’s or Biden’s presidency, Abbott stands to benefit.
Regardless of the winner of the 2020 election, Abbott remains a winner for as long as COVID-19 continues to threaten the world.
While the majority of COVID-19 vaccine companies have yet to generate income from their coronavirus programs due to pending FDA approvals, Abbott has been leveraging its pipeline to boost its growth even with the pandemic.
Since the early days of this health crisis, Abbott has been working to stay ahead of the pack.
To date, the company has at least seven COVID-19 tests with emergency use authorization from the FDA and are available in the market.
These tests, which boosted Abbott’s diagnostics sales by 39% in the third quarter, range from detecting active cases to identifying whether a person has been infected with the virus in the past.
The latest swab test to join Abbott’s growing lineup of COVID-19 products is called the antigen test and is designed to deliver results in as fast as 15 minutes and costs only $5.
To add convenience, this test is connected to a mobile to allow users to access their results right away.
Prior to the antigen test, Abbott launched a rapid detection test called BinaxNOW. This test can also return results on-site within 15 minutes. It has a free digital app, which sends users with negative results a “digital health pass” right on their phones.
When BinaxNOW was launched in August, the Trump administration spent $760 million for 150 million tests.
This company has supplied over 100 million COVID-19 tests and generated roughly $881 million in sales in the third quarter, up from the $615 million it reported in the second quarter.
Abbott is one of the safer stocks to own in the healthcare sector, with sales estimates for this company expected to grow by 14% in 2021 and 2022.
So far, Abbott shares have climbed 22% this year. Even amidst the pandemic, Abbott raised its full-year guidance for its earnings per share from $3.25 to $3.55.
While the company has been focused on its COVID-19 programs, this strategy is not a one-time deal.
On the contrary, the popularity of its COVID-19 testing kits serves as the much-needed door-opener for Abbott to expand its medical venues—an effort that generally takes years to develop.
For instance, its diabetes care segment alone managed to achieve a 26.9% year-over-year jump in sales to reach $843 million in the third quarter.
On top of that, Abbott has an incredibly diverse pipeline with over 100 new products across its different business units.
Abbott is a widely known dividend aristocrat, paying quarterly dividends consistently since 1924. It has a proven track record of solid performance and a carefully curated suite of businesses that promises future rewards.
At the rate the company is growing and the future projects it has in its pipeline, this dividend aristocrat would no doubt continue with this proud tradition of rewarding its investors generously.