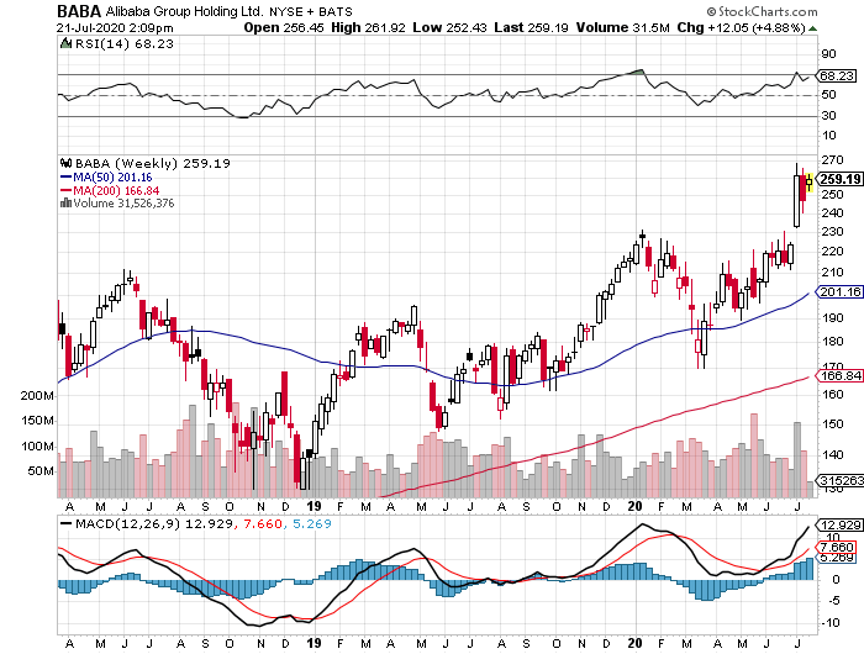
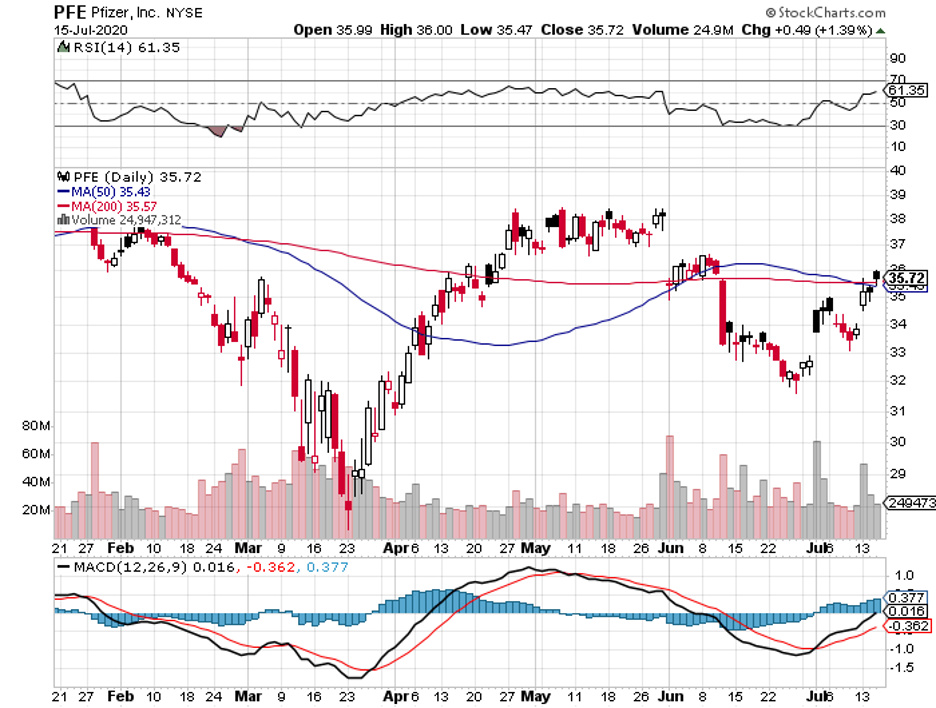
For investors, compounding has long been considered the eighth wonder of the world.
Compounding refers to growing your initial capital over time, boosted by well-timed additions to increase your pool of funds. Warren Buffet calls it “snowballing.”
For compounding to work out, though, it’s important to have a long-term investment plan.
Naturally, the first step to successfully invest is choosing the most suitable stock for your portfolio. Ideally, these businesses should have growth runways set up to thrive in the long run and coupled with clear-cut competitive advantages.
These companies should be able to pay out decent dividends as well since these can later be reinvested to accelerate returns.
Among the companies in the biotechnology and healthcare sector today, Bristol-Myers Squibb (BMY) fits the bill.
The company’s strengths lie in its oncology and hematology departments, with sales for its pipeline of drug candidates estimated to beat expectations.
With the new drugs in late-stage trials, BMY raised its annual sales forecast to reach somewhere between $15 billion and $20 billion. This number is expected to be sustained over the next 10 years.
Many of BMY’s promising programs came from its Celgene acquisition in November 2019.
While the whopping $74 billion deal faced pushback at first, the merger is expected to yield $2.5 billion in savings for BMY. This offers the company more elbow room to invest in its R&D sector.
BMY is working on combining its cancer drugs Opdivo and Yervoy with Celgene’s top moneymakers Revlimid and Pomalys, effectively transforming the New York-based company into the largest seller of cancer treatments in the world.
Outside its immuno-oncology lineup, BMY is also performing quite well in the cardiovascular field.
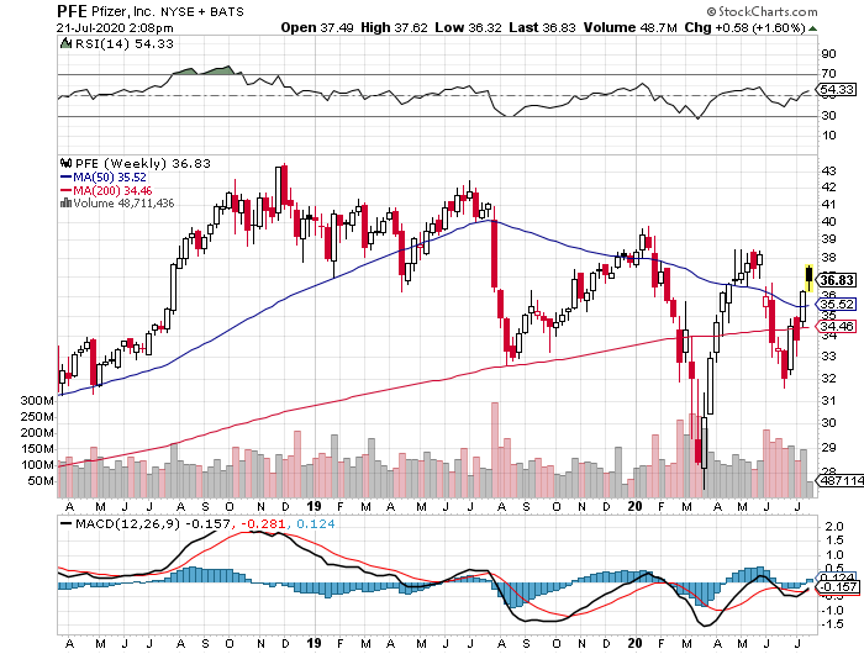
Its blockbuster drug Eliquis, which is a collaborative effort with Pfizer (PFE), remains one of the highest-selling treatments among atrial fibrillation patients.
In 2019 alone, Eliquis raked in $7.71 billion in sales. As for its performance this year, this heart disease drug is estimated to add another $1 billion, pushing its 2020 annual sales to $8.79 billion.
So far, 8 of BMY’s drugs available in the market generate over $1 billion in yearly sales. The company also has 9 new products undergoing Phase 3 trials, with more than 20 drugs slated for review in the next 10 years.
For 2020, BMY is projected to earn $41.8 billion in revenue and roughly $6.20 per share compared to $4.69 last year.
BMY is also anticipated to generate over $14 billion in free cash this year. Thanks to its Celgene acquisition, the company’s revenue will experience a one-time jump of about 60%.
For 2021, BMY is expected to report a 7.5% revenue growth to reach $45 billion or $7.33 per share. This is just a conservative estimate though.
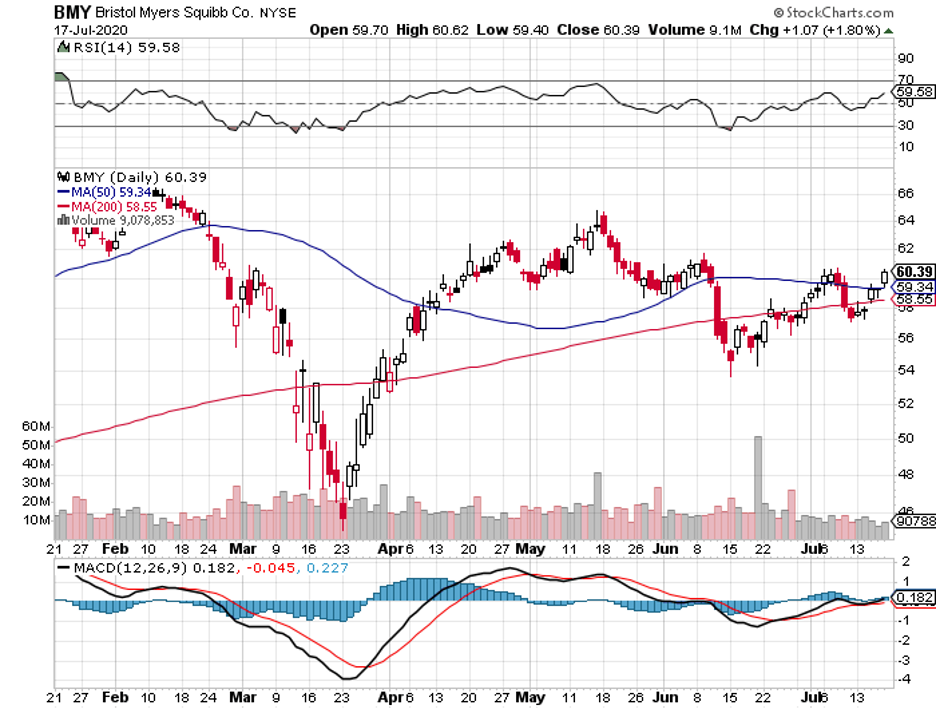
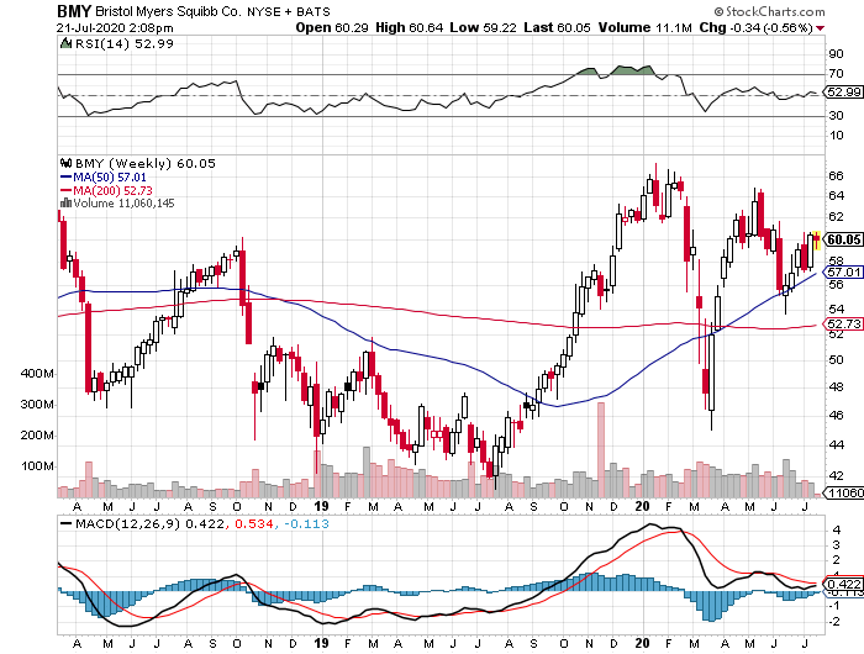
BMY is an attractive stock right now.
It’s currently trading at roughly $60. The company has about $136 billion in market capitalization and pays an annual dividend of $1.80 for a yield of 3%.
In the past 5 years, except for a single quarter in 2017, BMY reported positive quarterly earnings growth.
The shares trade for 8.6 times its expected earnings in the next 12 months, which is just ridiculous for a premium stock.
In terms of its long-term earnings per share, the company is expected to report a 9.3% growth rate.
Finding value among the biotechnology and healthcare sector has become increasingly tricky.
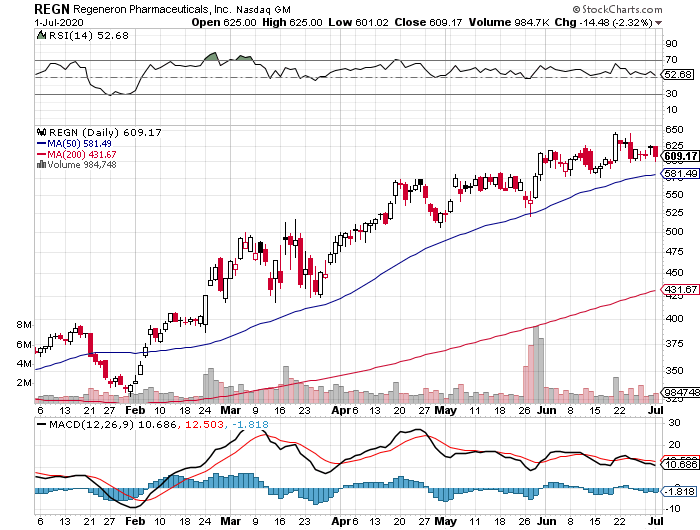
Since the pandemic broke, industry stalwarts like Gilead Sciences (GILD) and Regeneron Pharmaceuticals (REGN) have been receiving constant media attention for their COVID-19 vaccines and treatments. This pushed their valuations to skyrocket.
However, there remain a number of reasonably affordable biotechnology growth stocks.
While these are not making headlines in the fight against COVID-19, these companies offer attractively high long-term earnings-per-share growth rates – and BMY is one of them.