Mark your calendars because the world is about to find out whether the leading candidates of the COVID-19 vaccine race will be effective as early as October.
Other than saving lives in this pandemic, also hinged on the results is over $100 billion in investors’ money – an amount that reflects just how much value the stock market is putting on the COVID-19 vaccine candidates under development today.
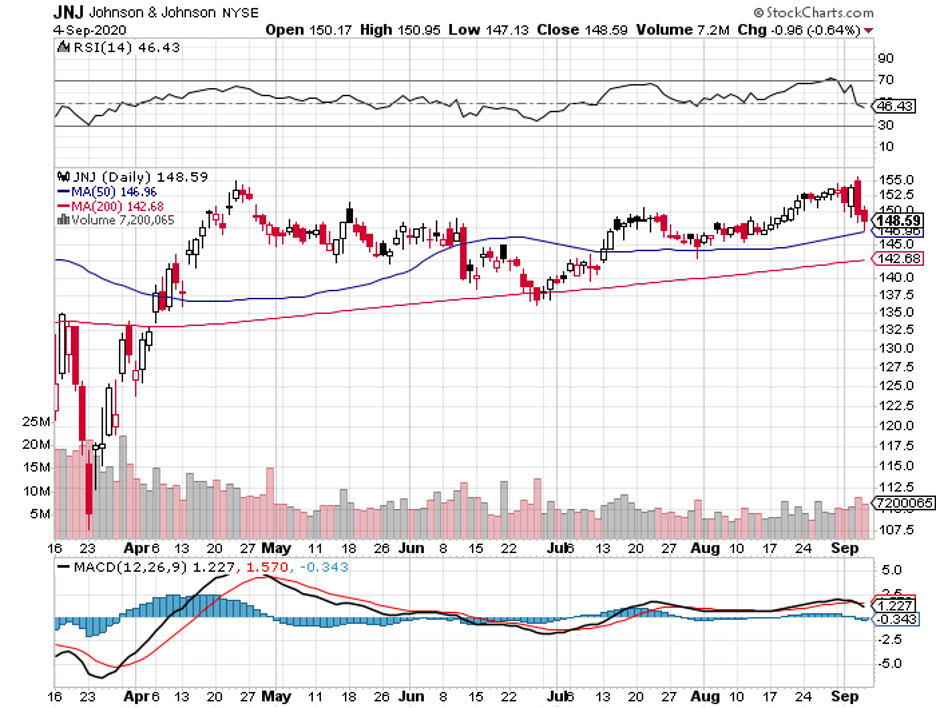
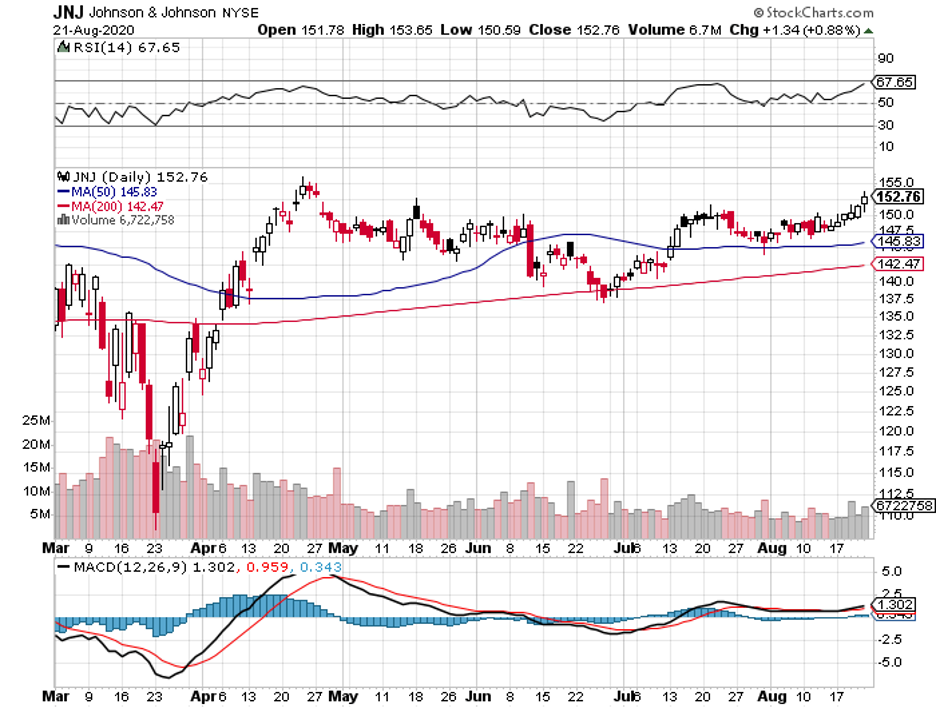
One of the leading companies in the race is Johnson & Johnson (JNJ).
With a market capitalization of almost $400 billion, many believe that this biopharmaceutical giant will soon be hailed as the king of the coronavirus stock list.
What we know so far is that JNJ’s subsidiary, called Janssen Vaccines, is set to launch a massive-scale Phase 3 study of its COVID-19 vaccine candidate, A26.COV2-S, this September.
The company’s study, which will be randomized, double-blind, and placebo-controlled, is expected to involve approximately 60,000 participants suffering from moderate to severe cases of COVID-19.
The move to include 60,000 participants is seen as a competitive advantage for JNJ because this is double the usual late-stage volunteer number.
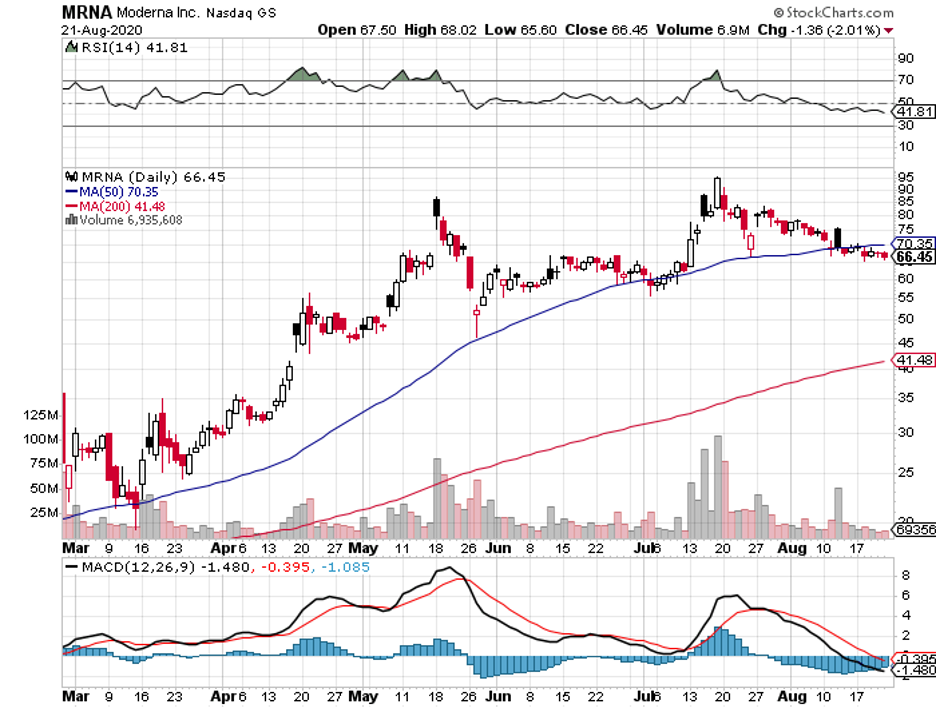
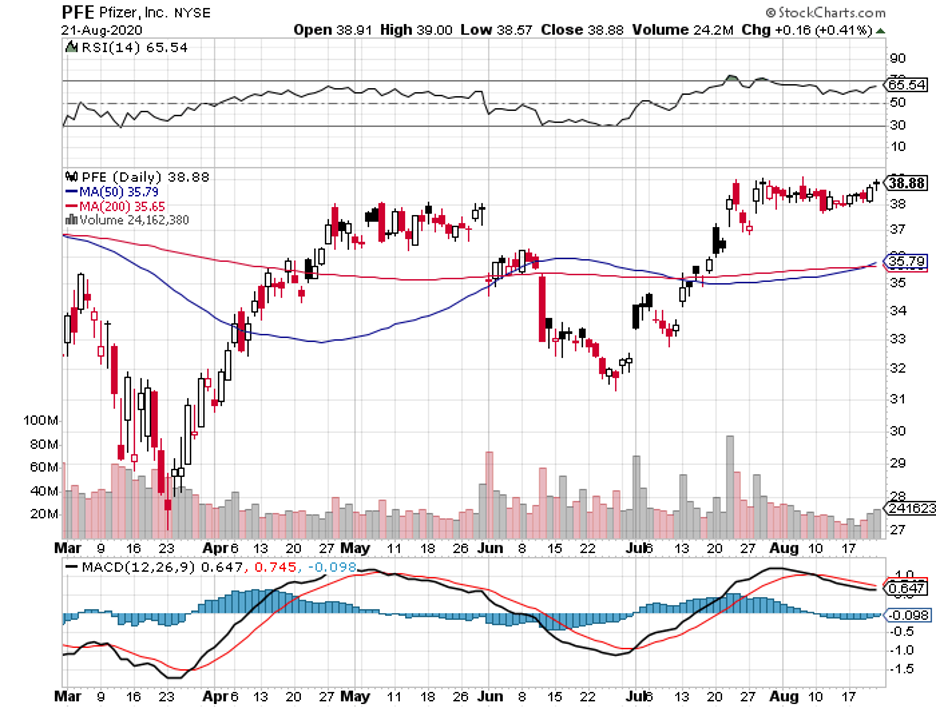
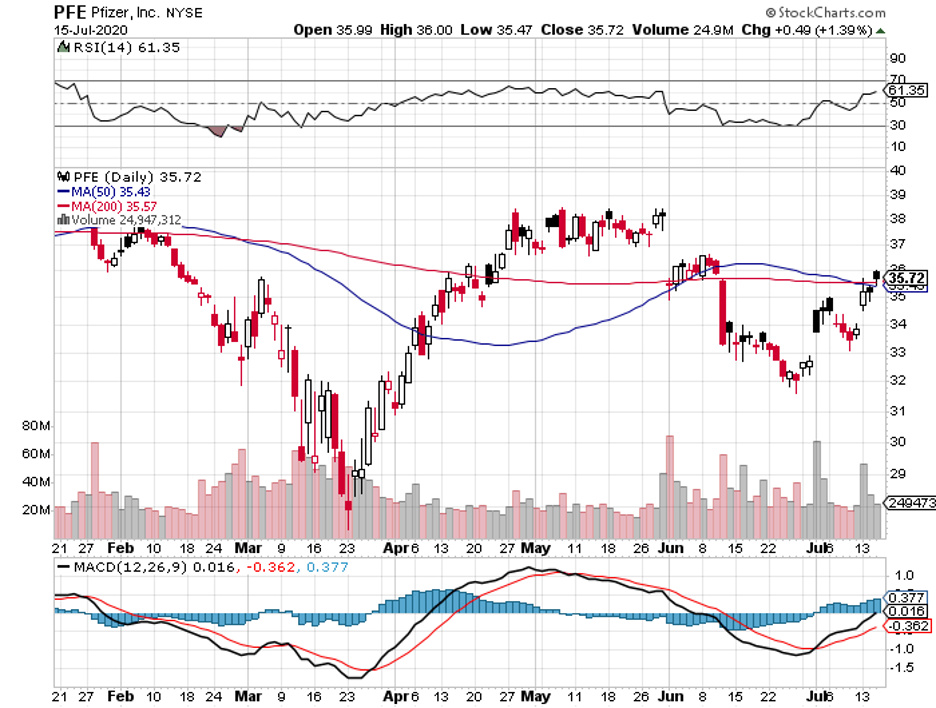
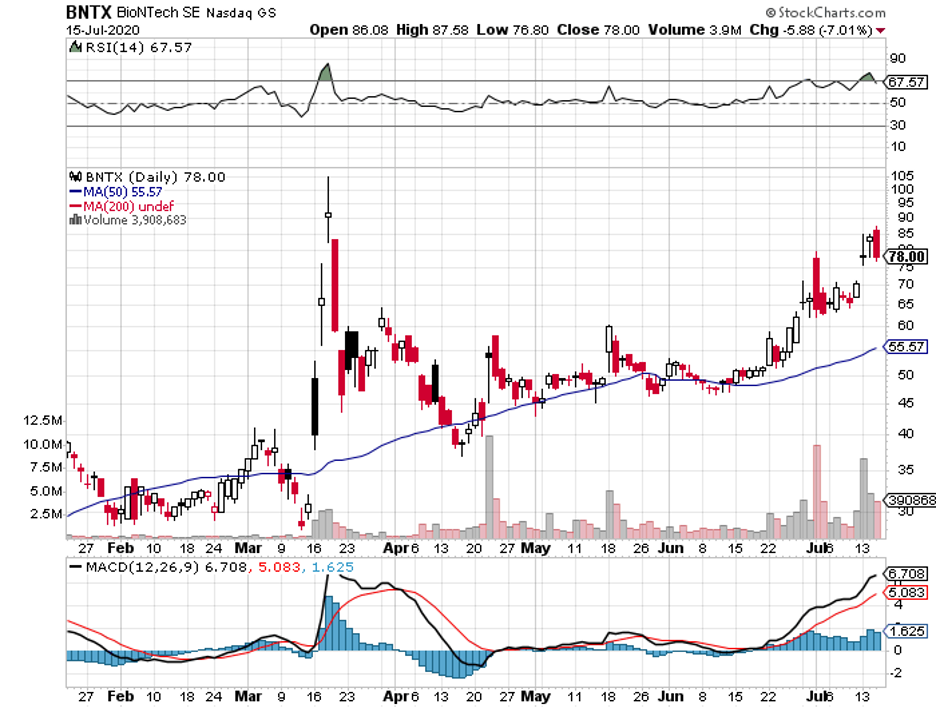
For comparison, Moderna’s (MRNA) mRNA-1273 as well as Pfizer (PFE) and BioNTech’s (BNTX) BNT162b2 will only target a maximum of 30,000 patients each in their trials.
On top of the more expansive trial coverage, JNJ has another advantage that could help it pull ahead of the pack.
During the preclinical testing of Ad26.COV2-S on primates, the vaccine candidate showed that a single dose could be enough to fight off the virus.
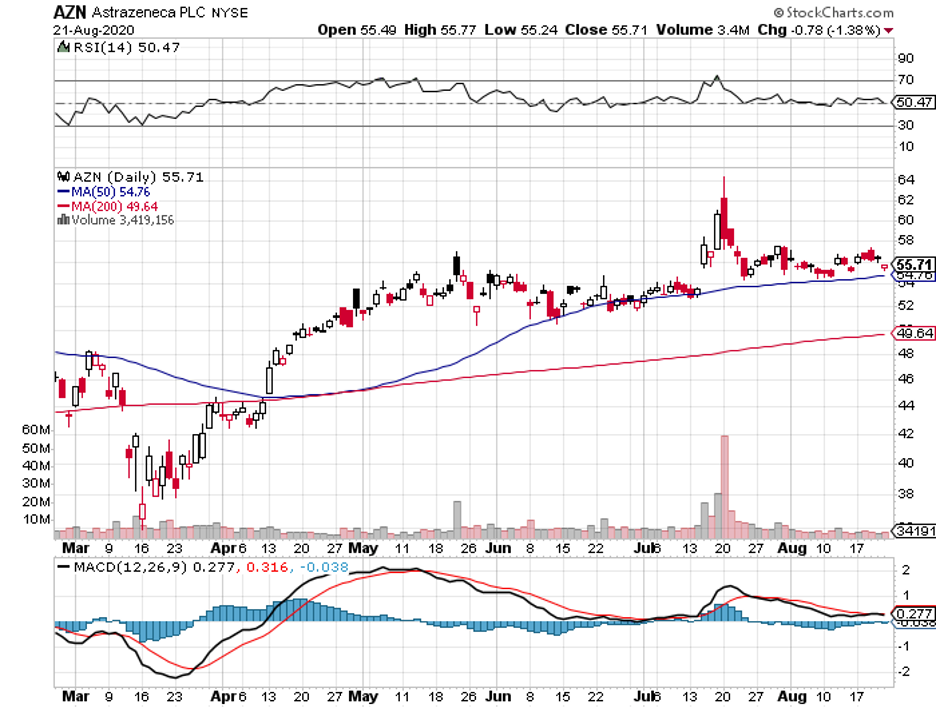
In contrast, the candidates from other COVID-19 vaccine developers like AstraZeneca (AZN), Pfizer, and Moderna require two doses to trigger a similar response.
While the well-being of every man, woman, and child hangs on the success of the COVID-19 trials, concerns have been raised that the assessment for these candidates might be compromised because of the upcoming US presidential election.
However, the leading COVID-19 developers assured people that it won’t be the case.
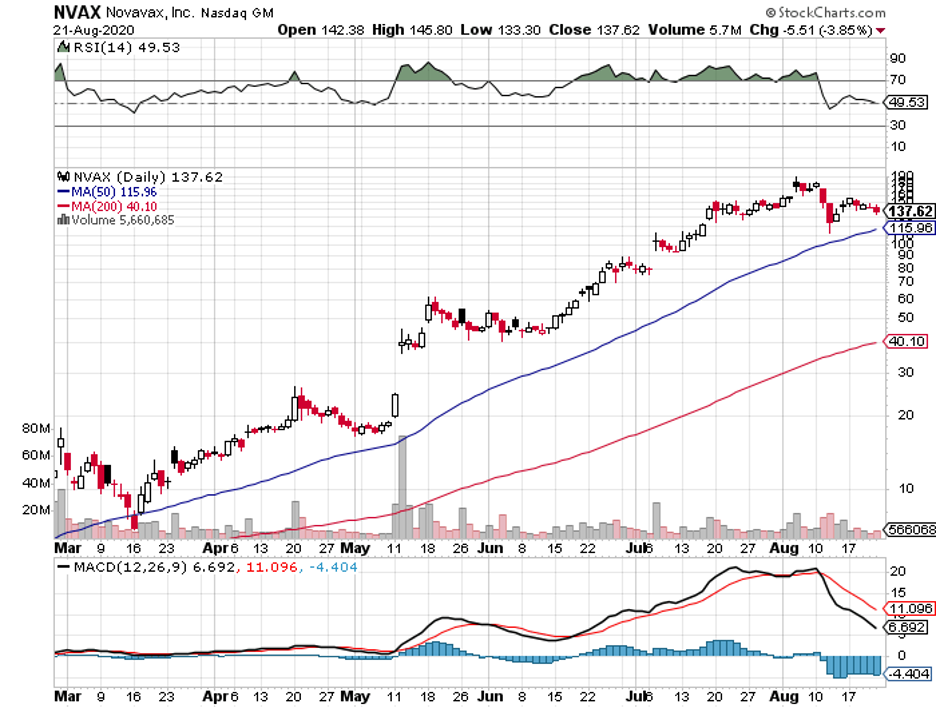
Apart from JNJ, Pfizer, Moderna, AstraZeneca, other vaccine makers like GlaxoSmithKline (GSK), Sanofi (SNY), and Regeneron (REGN) plan to issue statements that no candidate will be submitted without extensive data on its efficacy and safety.
Most investors are focused on the COVID-19 stocks these days, and who can blame them?
Sales of the vaccines in 2021 alone could reach $20 billion per company. This is massive profit even for Big Pharma standards.
In fact, this amount is higher than the projected sales of today’s current top-selling drug, Humira from AbbVie (ABBV), which is expected to clock roughly $19.6 billion in the same period.
However, the COVID-19 vaccines will only be profitable for as long as there is a pandemic. If this disease becomes a non-recurring sickness, then the vaccines will no longer be as profitable in the long run.
This is why it’s crucial to review the core operations of a company and determine its capacity to keep generating revenue and profits while also maintaining a strong balance sheet and returning value to its investors.
JNJ is a great example of this type of business.
Outside its COVID-19 efforts, the company has been diversifying its portfolio. Its latest move is the $6.5 billion acquisition of Momenta Pharmaceuticals (MNTA), marking the biopharmaceutical titan’s expansion into the immunology sector.
One of the most significant assets JNJ acquired from this deal is Nipocalimab, which is an incredibly promising first-in-class autoimmune disease treatment.
This drug could be the answer to rare and life-threatening blood disorders, such as hemolytic disease, which affects newborns and babies. To date, there are roughly 195 million individuals suffering from this condition worldwide.
Throughout its history, JNJ has proven itself to be a stable company even in the most unstable market conditions.
It has a reliable growth record and a healthy cash flow, which would be valuable in acquiring bolt-on companies, creating new drugs, and boosting the dividend every year.
JNJ has managed to increase dividends annually for 58 years now, with its most recent dividend raise reaching 6.3% just last April. Its stock currently yields 2.7%.
More importantly, JNJ offers an impressive biotechnology pipeline. With an incredible history of over 130 years, this stock is definitely one for keeps.